Abstract
Postictal neurologic deficit is a well-known complication mimicking the manifestation of a stroke. We present a case of a patient with clinical evidence of Todd's paralysis correlating with reversible postictal parenchymal changes on perfusion CT and magnetic resonance (MR) imaging. In this case, perfusion CT and MR imaging were helpful in the differential diagnosis of stroke-mimicking conditions.
Postictal neurologic deficit is a well-known complication of focal or generalized seizures mimicking clinical manifestation of a stroke; however, it can be misdiagnosed as a stroke (1-3). Thus, in an acute setting, imaging studies are the most useful means for making the correct diagnosis (3). To our knowledge, there were a few reports of cortical hyperperfusion during postictal motor deficit in the literature. We report a patient who was presented with postictal right hemiparesis accompanied by perfusion and diffusion abnormalities in the left parietal cortical areas. Our case suggests that Todd's paralysis may reveal transient perfusion and diffusion abnormalities; this report can be helpful for making an accurate diagnosis in patients with acute neurologic deficits.
A 72-year-old male was presented with sudden-onset right extremity weakness after clonic seizure. He had a history of nasopharyngeal cancer and his overall health condition was poor. He was alert and was able to follow commands. At initial neurologic examination, motor grade was III in the extremity of the right limb.
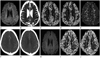
He underwent brain CT angiography (CTA), perfusion CT and magnetic resonance (MR) imaging including diffusion-weighted image (DWI) immediately after admission. On MR imaging (3T system, Achieva; Philips Medical Systems, Best, the Netherlands), an axial DWI showed subtle cortical hyperintensity in the left parietal lobe with restricted diffusion on an apparent diffusion coefficient (ADC) map (Fig. 1A, B). However, no abnormal finding was observed on other MR sequences. A perfusion CT (Somatom Definition AS+, Siemens Medical Solutions, Forchheim, Germany) demonstrated a dramatic increase in cerebral blood flow (CBF) and cerebral blood volume (CBV) involving mainly the cortical areas of the left parietal lobe with sparing white matter. Mean transition time (MTT), a sensitive indicator of acute cerebral ischemia, was relatively symmetric in configuration (Fig. 1C-E). CTA source images demonstrated slightly abundant vessels in the corresponding region with normal-appearing major intracranial arteries (Fig. 1F, G). Such findings suggested that acute neurologic deficit in this patient was unlikely to have been secondary to a vascular occlusive event. An electroencephalography was performed on the next day, and it demonstrated a diffuse left-hemispheric dysfunction without a definite epileptiform discharge.
However, a follow-up MR imaging on the same day revealed the disappearance of the preexisting diffusion abnormality (Fig. 1H). In addition, perfusion CT demonstrated normalization of CBF and CBV in the left parietal lobe (Fig. 1I, J).
The patient had no further seizures, and symptoms of the right hemiparesis gradually improved from grade III to IV over the next several days. Then, he was transferred to the medical department in order to treat acute gangrenous cholecystitis; no further neuroimaging was performed.
It is estimated that between 5 to 30% of cases identified as "brain attacks" are, in fact, due to stroke-mimicking conditions (3, 4). Stroke mimics include diagnosis such as complex migraine, infectious condition, metabolic disorder, intracranial tumor, epilepsy, Todd's paralysis and psychiatric illness, such as conversion disorder (3, 4). Despite the fact that Todd's paralysis is a well-known entity for postictal motor deficit, the physiologic explanation for it has not been established yet (2). Many mechanisms may account for the postictal state, including neurotransmitter depletion, neuronal desensitization, altered local cerebral blood flow and various forms of active inhibition, for which the utility of various imaging modalities have been investigated in the previous studies (2, 3, 5-8). However, the results of the previous studies regarding postictal perfusion findings are still controversial. Recently, Mathews et al. (2) demonstrated postictal regional hypoperfusion on perfusion CT caused by postictal exhaustion or inhibition, whereas Masterson et al. (3) reported regional hyperperfusion with postictal neurologic deficit due to the spread of a residual ictal discharge.
In this case, our patient's clinical and neuroimaging findings were consistent with those of the postictal state. We observed regional cortical hyperperfusion showing an increase in CBF and CBV with a relative preservation of MTT on the perfusion CT. According to previous studies, it is generally accepted that an increase in CBF and CBV is found in the seizure-onset zone as well as in the cortical areas affected by the spread of ictal discharges during the course of seizure, and the presence of a cortical hyperperfusion can be a valid indicator of ongoing seizure activity (3, 7, 9, 10). Further, there was increased vascularity in the corresponding region similar to the previous study with MR angiography (10). In addition, subtle cortical hyperintensity with lower ADC value on DWI showing normalization in the follow-up can be explained as a reversible excitotoxic brain injury mediated by prolonged seizure activity. The neuronal seizure activity increases the release of glutamate from the presynaptic terminals of neuronal axons, and excessive glutamate crosses the synaptic cleft in order to bind to the N-methyl-D-aspartate (NMDA) and non-NMDA receptors. This mechanism causes cytotoxic edema in neurons and adjacent glial cells, leading to apoptosis or selective neuronal necrosis. In this process, astrocytic response to excessive glutamate release plays an important role in tissue repair by dampening its excitotoxic effects. Therefore, cytotoxic edema in the acute phase of reactive astrocytosis is presumed to be responsible for reversible signal intensity abnormalities (6, 8).
In summary, our case provides a temporal and spatial correlation of regional cortical hyperperfusion in patients with postictal motor deficits. The transient nature of this clinical deficit correlates with the reversibility of cortical perfusion and diffusion abnormalities, and such findings can be helpful in diagnosing stroke-mimicking conditions.
Figures and Tables
Fig. 1
A 72-year-old male with acute onset aphasia and right extremity weakness.
A. Axial DWI shows subtle cortical hyperintensity in the left parietal lobe.
B. ADC reveals mild diffusion restriction.
C-E. Perfusion CT images demonstrate increased CBF (C) and CBV (D) in the left parietal cortices, but relatively normal configuration of MTT (E).
F, G. Axial CTA source images demonstrates mild increased vascular pattern.
H. Follow-up DWI on the next day shows no diffusion abnormality.
I, J. Follow-up perfusion CT at one week shows complete normalization of the preexisting hyperperfusion on CBF (I) and CBV (J) maps.
Note.-ADC = apparent diffusion coefficient, CBF = cerebral blood flow, CBV = cerebral blood volume, CTA = CT angiography, DWI = diffusion-weighted image, MTT = mean transition time
References
1. Davis DP, Robertson T, Imbesi SG. Diffusion-weighted magnetic resonance imaging versus computed tomography in the diagnosis of acute ischemic stroke. J Emerg Med. 2006; 31:269–277.
2. Mathews MS, Smith WS, Wintermark M, Dillon WP, Binder DK. Local cortical hypoperfusion imaged with CT perfusion during postictal Todd’s paresis. Neuroradiology. 2008; 50:397–401.
3. Masterson K, Vargas MI, Delavelle J. Postictal deficit mimicking stroke: role of perfusion CT. J Neuroradiol. 2009; 36:48–51.
4. Hemmen TM, Meyer BC, McClean TL, Lyden PD. Identification of nonischemic stroke mimics among 411 code strokes at the University of California, San Diego, Stroke Center. J Stroke Cerebrovasc Dis. 2008; 17:23–25.
5. Weinand ME, Carter LP, el-Saadany WF, Sioutos PJ, Labiner DM, Oommen KJ. Cerebral blood flow and temporal lobe epileptogenicity. J Neurosurg. 1997; 86:226–232.
6. Moritani T, Smoker WR, Sato Y, Numaguchi Y, Westesson PL. Diffusion-weighted imaging of acute excitotoxic brain injury. AJNR Am J Neuroradiol. 2005; 26:216–228.
7. Warach S, Levin JM, Schomer DL, Holman BL, Edelman RR. Hyperperfusion of ictal seizure focus demonstrated by MR perfusion imaging. AJNR Am J Neuroradiol. 1994; 15:965–968.
8. Chan S, Chin SS, Kartha K, Nordli DR, Goodman RR, Pedley TA, et al. Reversible signal abnormalities in the hippocampus and neocortex after prolonged seizures. AJNR Am J Neuroradiol. 1996; 17:1725–1731.
9. Wiest R, von Bredow F, Schindler K, Schauble B, Slotboom J, Brekenfeld C, et al. Detection of regional blood perfusion changes in epileptic seizures with dynamic brain perfusion CT--a pilot study. Epilepsy Res. 2006; 72:102–110.
10. Szabo K, Poepel A, Pohlmann-Eden B, Hirsch J, Back T, Sedlaczek O, et al. Diffusion-weighted and perfusion MRI demonstrates parenchymal changes in complex partial status epilepticus. Brain. 2005; 128(Pt 6):1369–1376.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download