Abstract
We report the potentials and limitations of computational fluid dynamics (CFD) analysis of patient-specific intracranial model with modification of proximal and distal length. Flow pattern does not seem to be affected by the length of proximal internal carotid artery. However, most of the flow was directed to the shorter distal part. Our study could serve as a technical reference to validating other tools and CFD results.
Computational fluid dynamics (CFD) is an important field in mechanical engineering. Since the 1950s, CFD has been widely utilized in various fields, not only in mechanical engineering, but also in aeromechanics, marine engineering, civil engineering, meteorological dynamics, environmental engineering, nucleonic and medical engineering. Navier-Stokes' equation is usually used for the analysis of viscous and compressible fluid, such as blood (1). With the advance of computational science, a non-linear Navier-Stokes' (NS) equation is easy to put into practice in CFD analysis instead of the linear NS equation, which converts the variable boundary conditions as simple constants. In contrast with the CFD assumption that the vessel wall is rigid and immovable, the fluid structure interaction, which is an integration of CFD with elastic walls such as human bodies, is also of interest in forensic medicine and medical engineering.
In particular, fluid dynamics of blood flow is relatively familiar to physicians and vascular surgeons, because it is usually used in studies on atherosclerosis or cerebral aneurysm.
Recently, CFD studies were published not only in the biomedical fields of which engineers comprise of the largest part, but also in the clinical fields where physicians play major roles. In many bio-medical journals, researchers have published papers regarding fluid dynamics associated with atherosclerosis and intracranial aneurysms. These studies were well-qualified technically, but they were not useful clinically because the studies were performed only in laboratories with time consuming in vitro models, special equipment and high budgets (2). On the other hand, attractive study results were reported on medical articles, but the methodological validations and discussions were usually omitted or inadequately described.
Due to the fact that many clinical studies used an in-house-developed software (3), most other researchers are now faced with difficulties in performing similar experiments. Only a few clinical studies have reported about CFD, except some medical engineering papers in Korea. We aimed to determine the potentials and limitation of CFD analysis in the actual clinical fields by using commercial programs with angiography equipment, which can provide a 3-dimensional (3D) imaging format to analyze the CFD.
We used the 3D vascular models for normal intracranial artery. Data acquisition and reconstruction of 3D vessel geometry came from 3D angiography, which was obtained using an AXIOM Artis Zee (Siemens Medical Solution, Erlangen, Germany) digital biplane angiography. The patients' identifying information was deleted from the data analysis. This study was approved by our Institutional Review Board after submission of an exemption form for informed consent.
Three steps of image post-processing are needed for the CFD analysis (Fig. 1). The first step is the segmentation for producing the 3D images, which generates the standard template library (STL) files in order to move on to the 2nd step processing. The source images could be obtained from CT, MRI and angiography. Until recently, commercial or individual laboratory programs have been used for performing this step in most studies, because it was not possible to generate an STL file with medical software. This work was time-consuming even for engineers with commercial programs who are not specialized for medical modeling. In our institution, this first step processing is performed using Syngo Workstation (Siemens Medical Solution, Erlangen, Germany) by the physician. The 3D angiographic images were transferred to Syngo Workstation in order to reconstruct the 3D angiography models with a large field of view. After a rough editing of unwanted vessels, the region not wanted for CFD analysis, 3D models were saved as a STL file. In our later studies, especially for intracranial vessels, Magics ver. 9.5.1 (Materialise, Leuven, Belgium) was used for surface editing (smoothing) of the vascular model. Fine editing of unwanted small perforators or branching vessels was performed. The original STL file was a 2-dimensional (2D) surface triangular mesh that is not suitable for fluid analysis due to its irregular triangular mesh of a twisted-section. We re-meshed evenly while preserving the original triangular mesh size of surface of the 2D vessel model. Files were saved as American Standard Code for Information Interchange STL for the next step.
The second step is the 3D volume meshing with Hypermesh (Altair Engineering, Inc., Auckland, New Zealand). We generated a 3D tetrahedral mesh after the correction processing consists of removal of the free edge, elimination of duplicated (overlapping) triangular mesh, removal of the irregular triangular mesh and normalization of the inner and outer surface of the triangular mesh plane. Files were saved as a nastran file for the next step. At the end of this step, we also generated the modified models for proximal and distal tubing (artificial extension), which were made for the evaluation of adequacy of CFD analysis for the original models.
The third step is the analysis. Computational analysis of blood flow in the blood vessel was performed using the commercial finite element and volume software ADINA version 8.6.2 (ADINA R & D, Inc., Lebanon, MA) using the NS equation. The number of tetrahedral elements in the 3D vessel model ranged from 100000 to 300000.
Blood flow was assumed to be laminar, viscous, Newtonian and incompressible due to its inherent flow characteristics. No-slip boundary conditions were assumed for the flow viscosity produced between the fluid and the wall surface of the blood vessels. Simulations were performed with the following material constants: the blood density was 1100 kg/m3 and blood dynamic viscosity was 0.004 Poiseuille. To achieve the truly patient-specific modeling, the boundary conditions at the inflow boundary were based on the pulsatile periodic flow rate. The unsteady flows in the internal carotid artery were computed over an interval of three cardiac cycles. We only applied the atmospheric conditions for the outlets. We used approximately 100000 to 300000 numbers of tetrahedral elements for CFD analysis, and the time step was 30 to 60 times per three cardiac cycles. Finally, we assumed the final cardiac cycle data as a result (4).
The ADINA module was also used for the post-processing of the analysis file. Blood velocity and eccentricity on the vascular cut surface, and wall shear stress in the blood vessels were analyzed. The velocity and flow rate of the internal carotid artery was calculated from the echo-cardiography gated phase contrast angiography without any intracranial vascular lesion using the Quantitative-flow software Viewforum version R 5.1 (Philips Medical Systems, Best, The Netherlands) (Courtesy of Dae Chul Suh, MD, Asan Medical Center).
It is essential to generate an STL file for CFD analysis. In additional to the extracranial 3D models, intracranial 3D models have a more irregular surface and further, it is difficult to make the very small vascular models for accurate analysis. An important first step of CFD analysis is extracting suitable CFD models for hemodynamic analysis. Removing the unwanted small vessels for CFD analysis is necessary. Furthermore, there are several obstacles in the vascular models, which makes it difficult for CFD analysis. Because of the smaller diameter of intracranial vessels compared to extracranial ones, a high-resolution source image is needed, usually obtained from conventional angiography (3) rather than CT or MRI.
There were several inappropriate factors for analysis in the surface triangular mesh of the generated STL files. Some 3D models showed a ragged mesh surface around the branch cutting; others presented an irregular and uneven size and shape of the triangle. Setting the boundary conditions specifying the vascular inlet, outlet and wall is one of the most important steps for CFD analysis. However, the cut surfaces of the vascular inlet and outlet seem to be ragged margins or not-perpendicular to the vessel wall. This pre-processing step is a very important process in CFD analysis, but time-consuming. Therefore, preprocessing is recognized as a very important step in CFD analysis, and more time and effort can be required than for the actual analysis.
The authors of this study attempted to resolve these problems; thus, the following processes were performed additionally. We use Magic's ver. 9.5.1 (Materialize, Leuven, Belgium) to edit the vessel walls and rimes, re-meshing and changing mesh density. We also use Hyper mesh (Altair Engineering, Inc., Auckland, New Zealand) for removing the free edges, eliminating the duplicated triangular meshes, and normalization, which assign the outer and inner walls of the vessel. After this process, we generated a 3D tetrahedral mesh and stored it as a nastran file. Because of the above factors, analysis of intracranial vessels could be possible on the intracranial segment only after the removal of the skull base and extracranial segment.
We could only use the laminar flow as an inlet condition instead of the fully developed flow due to technical limitations. Several studies reported that the proximal length of at least 20 times of the diameter is essential for the analysis of the fully developed flow. Thus, we transferred the elongated vascular model using Hypermesh. Two different conditions of the proximal to the region of interest, i.e., short vs. long internal carotid artery (ICA) model, show relatively similar CFD analytic effects. However, when we made the modified distal models with two different middle cerebral artery (MCA) lengths, the fluid flows converted more to a shorter distal portion (Fig. 2).
This may be the first study of CFD analysis led by physicians from the viewpoint of clinical practice in Korea. Clinical CFD analysis had been composed actively over the last 10 years, and CFD analysis of intracranial blood vessels has been conducted in only a few centers of foreign countries. In the biomedical engineering or engineering field, simplified or unmovable (fixed) models made from computer-aided design were used for fluid analysis (5). With a simplified model, all of the boundary conditions could be applied exactly. However, there are many problems, such as unclear boundaries, insufficient resolution or anatomical problems, in the analysis of in vivo vascular models, especially when using low-resolution MR or CT. Exact informative boundary conditions, such as flow velocity at outlets, are not well-recognized. There were no prior in vivo studies that mentioned them, especially the outlet conditions.
ADINA analysis took 30 minutes to 1 hour, but pre-processing using Magic and Hypermesh took much more time and also required a mechanical engineer's help. Post-processing with ADINA had also many limitations, because it is not dedicated for post-processing tools. Despite the many limitations and technical problems, CFD analysis in the clinical areas of the head and neck vascular systems is useful for the clinical assessment of risk factors in atherosclerosis of the carotid artery (6) or intracranial stenosis (4), risk of aneurismal rupture, and blood flow change before and after partial embolization (7) or flow diverting stents (8).
We performed CFD analysis of vascular models with different lengths of proximal and distal parts. Two different conditions of the proximal to the region of interest do not significantly affect the intracranial flow pattern. With that assumption, we hope that we reduce the time for CFD analysis using a shorter proximal model. Further accumulation and validation of other clinical models could be required for various disease models. When the distal length is changed differently, most of the flow was geared towards the shorter distal part with low resistance. This finding is also applied to clinical situations. For example, inappropriate length of anterior and MCA could affect the flow condition at distal ICA as well as lumen of posterior communicating artery aneurysm. For this reason, arbitrary removal of some branches or vessels distal to the region of interest can affect the CFD results. This problem should be overcome through further studies in order to utilize them in clinical practice.
The limitations of this study are as follows. First, all programs used in CFD analysis and preprocessing are expensive commercial engineering programs; some are academic packages and others needed cooperation with other institutes or laboratories. In this process, although collaboration between the physician and engineers was not easy, meeting many times as well as cooperation were conducted in spite of many limitations of time and space. Second, there were several new suggestions during cooperation with post-processing programs; however, these present financial problems. We hope the cooperation between industrial-educational institutes could improve the results.
This study is the first clinical trial of CFD in intracranial vessels in Korea. Through this study, we can obtain more information and limitations which may have been easily overlooked in recent published medical journals of CFD analysis regarding clinical disease models. However, further evaluation will be needed for CFD analysis tools in the clinical setting, and we believe that this study can provide the basis for clinical and technical considerations.
Figures and Tables
 | Fig. 1Work flow of computational fluid analysis.
Note.-CFD = computational fluid dynamics, STL = standard template library, 3D = 3-dimensional
|
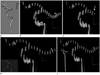 | Fig. 2Original and modified models shows changes of velocity at individual vessels.
A. Original 3-dimensional model. Computational fluid dynamics analysis shows velocity cut surface in systolic phase.
B. Original model.
C. Short middle cerebral artery (arrow) model.
D. Short proximal model.
E. Proximal elongated model
|
References
1. Milnor WR. Hemodynamics. 2nd ed. Baltimore, MD: Williams & Wilkins;1989.
2. Younis HF, Kaazempur-Mofrad MR, Chan RC, Isasi AG, Hinton DP, Chau AH, et al. Hemodynamics and wall mechanics in human carotid bifurcation and its consequences for atherogenesis: investigation of inter-individual variation. Biomech Model Mechanobiol. 2004; 3:17–32.
3. Cebral JR, Mut F, Weir J, Putman CM. Association of hemodynamic characteristics and cerebral aneurysm rupture. AJNR Am J Neuroradiol. 2011; 32:264–270.
4. Suh DC, Park ST, Oh TS, Park SO, Lim OK, Park S, et al. High shear stress at the surface of enhancing plaque in the systolic phase is related to the symptom presentation of severe M1 stenosis. Korean J Radiol. 2011; 12:515–518.
5. Nakatani H, Hashimoto N, Kang Y, Yamazoe N, Kikuchi H, Yamaguchi S, et al. Cerebral blood flow patterns at major vessel bifurcations and aneurysms in rats. J Neurosurg. 1991; 74:258–262.
6. Park ST, Kim JK, Yoon KH, Park SO, Park SW, Kim JS, et al. Atherosclerotic carotid stenoses of apical versus body lesions in high-risk carotid stenting patients. AJNR Am J Neuroradiol. 2010; 31:1106–1112.
7. Goubergrits L, Thamsen B, Berthe A, Poethke J, Kertzscher U, Affeld K, et al. In vitro study of near-wall flow in a cerebral aneurysm model with and without coils. AJNR Am J Neuroradiol. 2010; 31:1521–1528.
8. Cebral JR, Mut F, Raschi M, Scrivano E, Ceratto R, Lylyk P, et al. Aneurysm rupture following treatment with flow-diverting stents: computational hemodynamics analysis of treatment. AJNR Am J Neuroradiol. 2011; 32:27–33.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download