Abstract
We report a 46-year-old woman patient with reversible cerebral vasoconstriction syndrome (RCVS). She presented with severe headache, multiple cerebral infarction, and multifocal severe stenosis in the intracranial arteries on magnetic resonance angiography (MRA). One month after the episode, a small bowel gastrointestinal stromal tumor (GIST) was incidentally detected during the evaluation of severe anemia and GIST was removed. Follow-up MRA was performed 3 months and 1 year after an initial attack of headache, and multifocal severe intracranial arterial stenotic lesions were completely resolved, she did not experience any episode of RCVS during the 2 years.
Reversible cerebral vasoconstriction syndrome (RCVS) is characterized by the association of severe headaches with or without focal neurological deficit, and a reversible cerebral vasoconstriction assessed by an initial angiography (MR, CT or conventional angiography), with the disappearance of arterial abnormalities within less than 3 months demonstrated by a follow-up angiography (1). RCVS usually occurs in middle-aged women. Recurrent thunderclap headaches, defined as severe headaches peaking at less than 1 min, are the clinical hallmark of RCVS (1-4). Postpartum, exposure to vasoactive drugs, and catecholamine-secreting tumors are well known precipitating causes of RCVS (1). We report a RCVS case of a 46-year-old woman patient. In this case, the precipitating cause for RCVS was not disclosed; however, a small bowel gastrointestinal stromal tumor (GIST) with gastrointestinal (GI) bleeding was incidentally detected. This report describes the clinical and imaging features of this case, and the questionable relationship between RCVS and GI bleeding will be briefly discussed.
A 46-year-old previously healthy woman experienced sudden severe headache (visual analog scale score = 7) with vomiting and a sensation of a hammer beating inside the head. She visited the hospital and received a brain CT scan. The CT showed no abnormality, and she was conservatively managed with analgesics. However, she experienced a waxing and waning headache every other day. 17 days after the first episode of headache, a sudden right side low extremity weakness developed, and she was admitted to a tertiary hospital. Neurological examination revealed a right lower-extremity monoplegia (grade 2) and sensory loss to pain and touch in the right lower extremity. Otherwise, her vital signs and the results of physical and laboratory examinations, except for the value of hemoglobin and hematocrit, were within a normal range. Magnetic resonance imaging (MRI) revealed a subacute left side anterior cerebral artery (ACA) and right posterior cerebral artery territory (PCA) infarction with hemorrhage (Fig. 1). Magnetic resonance angiography (MRA) revealed bilateral multifocal severe stenosis and a beaded appearance in ACA, middle cerebral artery (MCA), PCA, distal vertebral artery, and basilar artery (Fig. 1). She was conservatively managed with aspirin and Gliatilin (choline alfoscerate), and her right side weakness and sensory loss were improved. During admission, anemia was detected with a fluctuating hemoglobin level (hemoglobin level 8.9→8.3→ 7.4→10.4 g/dL) over 3 days; this was conservatively managed with blood transfusion. She was discharged and received a rehabilitation treatment in a local rehabilitation hospital. During a rehabilitation treatment, she experienced a syncope and dizziness, and severe anemia was detected (hemoglobin 6.4 g/dL, hematocrit 21.1%). She was admitted to our hospital for evaluation of anemia 1 month after the previous ischemic event. Endoscopy for stomach, duodenum, rectum, and colon was normal. Enhanced CT scan revealed a 6.9 cm sized solid enhancing mass abutting small bowel loop in the lower abdomen, and a small bowel GIST or lymphoma was suspected. The mass was excised and diagnosed as a GIST with a low mitotic index. After the operation, she recovered well and her hemoglobin level was elevated to 12.4 g/dL at the time of discharge. A follow-up MRA was performed 3 months after the initial attack of headache, multifocal severe stenosis of intracranial arteries was completely resolved, and the intracranial arteries appeared as normal (Fig. 2). Follow-up MRA was repeated 1 year after the 2nd MRA examination, and the intracranial arteries appeared as normal. During the 2 year follow-up period, she experienced no episode of RCVS since the removal of GIST.
Initial presentation of RCVS is usually a severe headache, and a moderate intermittent headache can develop between the episodes of severe headache. All significant headaches usually disappear 3 weeks after the initial attack of headache (1). Imaging abnormalities in RCVS include convexity subarachnoid hemorrhage (SAH), reversible brain edema, intracerebral hemorrhage (ICH), and cerebral infarction (2-4). In all cases, reversible vasoconstriction of cerebral arteries should be documented by angiographic imaging (1). Imaging abnormalities on CT or MRI have been reported in 37-81% of RCVS patients, and a combination of lesions can be present (3, 4). Convexity SAH and reversible edema are early imaging manifestations and usually can be seen within the first week of headache onset. Convexity SAH has been reported in 30-34% of RCVS patients and it is seen in a few cases of sulci near convexity (3, 4). Reversible brain edema has been reported in 8-38% (2-4) and it may have a similar distribution to that of posterior reversible encephalopathy syndrome (2-4). ICH has been reported in 12-20% of RCVS patients (3, 4) and it is more frequently single than multiple and more lobar than deep, and is usually associated with other types of abnormalities such as convexity SAH or infarction (3, 4). ICH can occur early in the course of RCVS (3). Ischemic strokes have been reported in 6-39% of RCVS patients (2-4) and they usually occur later in the course of RCVS. In the previous reports, infarctions usually occurred about 10 days after the first thunderclap headache (2-4). For diagnosis of RCVS, a cerebral angiography is needed to show segmental narrowing and dilatation (string of beads) of one or more intracranial arteries (1). The initial angiogram may be normal if it is performed within one week of the initial attack. In such cases, a second angiogram several days later may be diagnostic (2). Maximum vasoconstriction of the branches of the MCAs is reached at a mean of 16 days after clinical onset (2). Although no pathophysiology has been established, the unpredictable and transient dysregulation of cerebral arterial tone with sympathetic overactivity seems to have a role in the development of RCVS (1). Precipitants of RCVS are various and include the postpartum period, vasoactive drugs such as cannabis, cocaine, antidepressants, nasal decongestants, and ergot alkaloid, catecholamine-secreting tumor such as pheochromocytoma, glomus or carcinoid tumor, and immunosupressants or blood products such as transfusion (1, 5, 6). Although the exact precipitating cause was not determined, GI bleeding from GIST might be a possible precipitant of RCVS in our case. GI bleeding or GIST was not associated with RCVS in the previous reports. Paraneoplastic hypoglycemia induced by an insulin-like growth factor II from GIST has been reported in some patients (7). However, there have been no reports of any GIST producing catecholamine. In this case, we did not evaluate the level of blood catecholamine. Therefore, there may not be sufficient evidence to suggest that GIST may be a precipitant of RCVS. In our opinion, GI bleeding might be the precipitating cause of RCVS. Although the hemoglobin level at the initial attack of headache was not available, the available data of the hemoglobin level suggested that intermittent and considerable GI bleeding might have been present before the event of RCVS. Intermittent and considerable GI bleeding may induce an increased sympathetic tone by sympathetic reflex compensation (8). Therefore, we presume that GI bleeding from the GIST may be a possible precipitant of RCVS in this patient.
The differential diagnosis of RCVS is cerebral vasculitis, including primary angiitis of central nervous system and vasospasm induced by aneurysmal SAH (a-SAH). The initial presentation of this case mimicked a-SAH; however, the amount of SAH was not sufficiently significant to suspect aneurysm rupture, and the clinical course completely differed to that of a-SAH. Vasculitis may mimic the vasospasm and parenchymal changes of RCVS. Usage of steroids is usually avoided in RCVS because steroids may worsen the clinical course of RCVS (4, 9). Therefore, differential diagnosis with cerebral vasculitis may be important. In cerebral vasculitis, the headache is usually not severe and depending on the clinical course, with the thunderclap type headache, the patient may have recurrent episodes of attack. In vasculitis, arterial abnormalities do not improve as rapidly (1).
In conclusion, we report a RCVS case in a patient with GI bleeding from a small bowel GIST. Although the causality for RCVS may not be definite, considerable GI bleeding from GIST may be a suspicious precipitant of RCVS in this case.
Figures and Tables
Fig. 1
MR imaging features of RCVS in a 46-year-old woman.
A-E. Diffusion-weighted imaging (A), apparent diffusion coefficient map (B), and FLAIR (D, E) show early subacute infarctions in the right PCA and left ACA territories. T2*-gradient echo imaging (C) shows early subacute ICH in the right occipital lobe. FLAIR (E) shows subtle sulcal hyperintensity (arrow) that is suspicious for convexity SAH.
F. Time-of-flight MRA shows multifocal segmental severe narrowing with or without adjacent segmental dilatation in bilateral MCA, ACA, PCA, basilar artery, and distal vertebral artery.
Note.-ACA = anterior cerebral artery, FLAIR = fluid attenuated inversion recovery, ICH = intracerebral hemorrhage, MRA = magnetic resonance angiography, PCA = posterior cerebral artery, RCVS = reversible cerebral vasoconstriction syndrome, SAH = subarachnoid hemorrhage
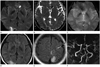
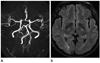
Fig. 2
Follow-up MR imaging 3 months after initial attack of RCVS.
A. Multifocal severe stenosis disappears on time-of-flight MRA and intracranial arteries looks as normal.
B. FLAIR imaging shows resolution of the lesions with subtle residual high signal intensity in the infarction area and low signal intensity in the ICH area.
Note.-FLAIR = fluid attenuated inversion recovery, ICH = intracerebral hemorrhage, MRA = magnetic resonance angiography, RCVS = reversible cerebral vasoconstriction syndrome
References
1. Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012; 11:906–917.
2. Chen SP, Fuh JL, Wang SJ, Chang FC, Lirng JF, Fang YC, et al. Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann Neurol. 2010; 67:648–656.
3. Ducros A, Fiedler U, Porcher R, Boukobza M, Stapf C, Bousser MG. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke. 2010; 41:2505–2511.
4. Singhal AB, Hajj-Ali RA, Topcuoglu MA, Fok J, Bena J, Yang D, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011; 68:1005–1012.
5. Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007; 146:34–44.
6. Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007; 130(Pt 12):3091–3101.
7. Escobar GA, Robinson WA, Nydam TL, Heiple DC, Weiss GJ, Buckley L, et al. Severe paraneoplastic hypoglycemia in a patient with a gastrointestinal stromal tumor with an exon 9 mutation: a case report. BMC Cancer. 2007; 7:13.
8. Guyton AC, Hall JE. Textbook of medical physiology. 11th ed. Philadelphia: Elsevier Saunders;2006. p. 279–281.
9. French KF, Hoesch RE, Allred J, Wilder M, Smith AG, Digre KB, et al. Repetitive use of intra-arterial verapamil in the treatment of reversible cerebral vasoconstriction syndrome. J Clin Neurosci. 2012; 19:174–176.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download