Abstract
Post-radiation malignant fibrous histiocytoma (MFH) of the breast is extremely rare. We report a case of post-radiation MFH that presented a rapidly growing mass in a 52-year-old woman who underwent breast-conserving therapy and adjuvant whole breast irradiation 6 years ago. To the best of our knowledge, only one case of primary MFH of the female breast have been reported with sonographic findings. We analyzed the sonographic and MRI findings with correlative histopathologic features, and then confirmed with surgical excision.
Radiation induced sarcomas (RIS) are rare complications of radiotherapy that occur from a previously irradiated field after several years. Angiosarcoma is the most common sarcoma after radiotherapy for breast carcinoma, and malignant fibrous histiocytoma (MFH) is extremely rare among the RIS (1).
We report a case of post-radiation MFH which presented a rapidly growing mass in a 52-year-old woman who previously underwent breast conserving therapy and adjuvant radiotherapy.
A 52-year-old woman with a rapidly growing mass in her right breast was admitted to the breast clinic center. She had undergone a partial mastectomy for her upper right outer breast and a sentinel lymph node biopsy 6 years ago. The tumor was a 2.7 × 2.5 cm invasive ductal carcinoma without lymph node metastasis (T2N0M0). Immunohistochemistry was negative for the estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2. Following the adjuvant chemotherapy, she received adjuvant radiation therapy (RT) for 6 weeks. The dose delivered to the whole breast was 5040 cGy in 28 fractions, and the boost dose to the primary tumor bed was 1600 cGy in eight fractions. She tolerated her radiation treatments, and no specific complications were observed during RT.
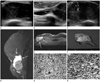
Sixty-nine months after the initial therapy, she noticed a lump on the inframammary fold of her right breast. Physical examination revealed a bulging hard mass in the right inframammary fold area. Ultrasound was performed by using a 5-12 MHz linear probe (IU22 Ultrasound system, Philips Ultrasound, Bothwell, WA, USA). The mass was 6 × 2.8 cm in size with a circumscribed margin, it was relatively oval in shape, and showed complex echogenicity and elongated septated cystic area on the upper portion of mass at the right inframammary fold area (Fig. 1A, B). On color Doppler ultrasonogram, the mass showed some vascularity within the mass (Fig. 1C). The patient did not perform mammography due to pain during compression of the infected breast. The MRI (1.5T, Signa Excite HDx, GE Healthcare, Milwaukee, WI, USA) demonstrated a solid and cystic tumor invading the pectoralis major muscle. On T2 weighted sagittal images, the mass had well defined margin, an oval shape and high signal intensity with peripherally high signal intensity cystic area (Fig. 1D). On post-contrast T1 weighted images, the mass showed inhomogeneous intense enhancement with peripheral cystic area and irregular enhancement obliterating fat plane between the pectoralis muscle, suggesting an invasion of pectoralis muscle (Fig. 1E). This intensive, inhomogeneous enhancement presented a washout pattern on post-contrast T1-weighted images of delayed phase by visual inspection. There was no axillary lymphadenopathy on magnetic resonance imaging (MRI). The tumor was not found on the MRI that had been performed 6 months ago.
We performed ultrasonography-guided core biopsy of the mass, and the pathologic result was spindle-shaped, fibroblast-like cells with atypicality and mitosis (Fig. 1F). Wide excision of the breast and chest wall including the fifth and sixth ribs was performed. The pathologic diagnosis was MFH with the pectoralis muscle invasion (Fig. 1G, H). There was no node metastasis on pathologic examinations and all resection margins were negative.
Breast-conserving therapy, consisting of lumpectomy and RT, is a successful, well-studied, and scientifically validated treatment of early-stage breast cancer. Multiple prospective, randomized trials have demonstrated that adjuvant RT decreases the risk of local disease recurrences and increases the rate of breast preservation (2). Because the number of patients treated with conservative surgery and radiation therapy has been increasing, the incidence of treatment-associated soft tissue sarcoma has increased over time (3). RIS was first described in the setting of radical mastectomies, and was later found in patients undergoing lumpectomy and radiation therapy (4). RIS, a rare iatrogenic malignancy, can occur after radiotherapy and is associated with poor outcomes. After radiotherapy, the cumulative RIS incidence is 3.2 per 1000 at 15 years (versus 2.3 per 1000 for primary sarcoma in a population without radiotherapy) (5).
The incidence of sarcomas in breast cancer patients following mastectomy and chest wall irradiation is reported to be 0.2% at 10 years (6). Post-radiation sarcoma accounts for 0.5% to 5.5% of all sarcomas and the most common RIS location is the chest wall (7). Angiosarcoma, malignant fibrous histocytoma, and leiomyosarcoma are the most common histological RIS subtypes following after breast cancer (1, 3). Typically, the radiation-induced angiosarcoma (RIA) of the breast appears in cutaneous areas. The differential diagnosis for RIA includes inflammatory breast carcinoma, edema of the breast, and cellulitis (8).
MFH is the most common adult soft tissue sarcoma in the deep connective tissues of the extremities, abdominal cavity and retroperitoneum. In the extremities, the sonographic appearance of MFH is usually the inhomogeneous hypoechoic mass with areas of necrosis. MFH of the breast has similar sonographic findings as with the other organs. In reported case of primary MFH on the breast, it showed well-demarcated solid and cystic mass with increased blood flows in the solid component on Doppler ultrasound (9). Biswas and Badiuddin (4) reported mammographic findings of the radiation-induced MFH for contralateral breast following the treatment of breast cancer. The mammographic finding was of irregular high density mass with skin tethering.
To our knowledge, sonographic and MRI findings of post-radiation MFH of the breast have not yet been reported. Our case showed a circumscribed, oval mass with elongated septated cystic area on ultrasound images. The echogenicity of the mass was complex, with hypervascularity in the solid component. The previous report described such cystic areas as sites of necrosis or hemorrhage (9). Cystic areas have strong signal intensity on T2-weighted images and no contrast enhancements on enhanced MR images. The solid mass showed inhomogeneous enhancements with washout patterns on post-contrast T1-weighted images, similar to the usual breast carcinoma. In addition, findings of pectoralis muscle invasion are well displayed on MRI.
The imaging features of post-radiation sarcomas are not pathognomonic. In addition to imaging features, a low incidence after long latency period makes it difficult to diagnose the disease accurately. MRI can help facilitate excellent preoperative planning for the extent of resection. MRI scans can reveal the spread of a tumor and predict chest wall involvements, by facilitating operative intervention (7, 10). The 5-year survival rate of RIS patients is in the range of 27-48%. The number of skin lesions is an important prognostic factor (1, 5). The standard treatment is surgery, specifically wide local excisions with negative surgical margins.
In conclusion, we report the first case of post-radiation MFH following the treatment of breast cancer with findings from sonography and MRI. We suggest that MFH should be considered in the differential diagnosis of a bizarre mass in the breast with progressive growths within a short period of time, especially following the treatment breast cancer.
Figures and Tables
Fig. 1
A 52-year-old woman with a rapidly growing mass in her right lower breast near the inframammary fold.
A. Ultrasonography reveal a 6 × 2.8 cm oval, circumscribed mass with a complex echogenicity at the 6 o'clock position.
B. Elongated septated cystic area can be seen at the upper portion of mass (arrows).
C. On color Doppler sonography, there is some vascular flow within the mass.
D. A sagittal, fat-saturated, T2-weighted MR image shows an approximately 6 cm mass with high signal intensity, and peripheral portion of the mass showed avidly high signal intensity, which suggested cystic area (arrows).
E. A post-contrast fat-suppressed T1 weighted MR axial image demonstrate a inhomogeneous enhancing solid tumor with surrounding non-enhancing cystic area invading the pectoralis major muscle at the right lower inframammary fold area (arrows).
F. A yellow solid tumor was totally excised along with the pectoralis muscle and ribs.
G. Microscopic features consisting of plump spindle cells and pleomorphic cells with mitotic activity (hematoxyline and eosin stain, original magnification × 200).
H. Immunohistochemical staining demonstrating diffuse cytoplasmic positivity of tumor cells (Immunohistochemistry, original magnification × 200).
References
1. Kirova YM, Vilcoq JR, Asselain B, Sastre-Garau X, Fourquet A. Radiation-induced sarcomas after radiotherapy for breast carcinoma: a large-scale single-institution review. Cancer. 2005; 104:856–863.
2. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–1241.
3. Mery CM, George S, Bertagnolli MM, Raut CP. Secondary sarcomas after radiotherapy for breast cancer: sustained risk and poor survival. Cancer. 2009; 115:4055–4063.
4. Biswas S, Badiuddin F. Radiation induced malignant histiocytoma of the contralateral breast following treatment of breast cancer: a case report and review of the literature. Cases J. 2008; 1:313.
5. Yap J, Chuba PJ, Thomas R, Aref A, Lucas D, Severson RK, et al. Sarcoma as a second malignancy after treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2002; 52:1231–1237.
6. Pendlebury SC, Bilous M, Langlands AO. Sarcomas following radiation therapy for breast cancer: a report of three cases and a review of the literature. Int J Radiat Oncol Biol Phys. 1995; 31:405–410.
7. Sheppard DG, Libshitz HI. Post-radiation sarcomas: a review of the clinical and imaging features in 63 cases. Clin Radiol. 2001; 56:22–29.
8. Sheth GR, Cranmer LD, Smith BD, Grasso-Lebeau L, Lang JE. Radiation-induced sarcoma of the breast: a systematic review. Oncologist. 2012; 17:405–418.
9. Yao MS, Chan WP, Chen CY, Chu JS, Hsieh MC. Malignant fibrous histiocytoma of the female breast: a case report. Clin Imaging. 2005; 29:134–137.
10. Noh JM, Huh SJ, Choi DH, Park W, Nam SJ. Two cases of post-radiation sarcoma after breast cancer treatment. J Breast Cancer. 2012; 15:364–370.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download