Abstract
Sarcomatoid carcinomas are rare biphasic tumors composed of mixed malignant epithelial and mesenchymal cells. A few cases for small intestinal sarcomatoid carcinoma were reported. Moreover, most of the cases are focused on the pathologic review. We experienced a case of monophasic sarcomatoid carcinoma arising in the jejunum in a 78-year-old man. In this case, CT showed fungating mass with central necrosis in the jejunum. We also reviewed literatures on sarcomatoid carcinoma that arises in the small intestine.
By definition, sarcomatoid carcinomas are malignant tumors that exhibit histological characteristics of a combination of malignant epithelial and mesenchymal cells. These tumors can occur in various anatomical sites, including lungs, thyroid gland, salivary gland, breast, bladder, prostate, skin and digestive system (1). Gastrointestinal sarcomatoid carcinomas are unusual tumors that occur most frequently in the stomach, gallbladder, and esophagus (1). These are especially rare in the small intestine, with an incidence of only 0.5-0.8 per 100000 population per year (2). To the best of our knowledge, only 26 cases have been reported in the English literature up to date (3-8). Moreover, most of the cases are focused on the pathologic reviews.
Therefore, we present a case of sarcomatoid carcinoma arising from small intestinal tract with findings of the multi-detector computed tomography (MDCT) scans and reviews of the literature.
A 78-year-old man was presented at our hospital with complaints of intermittent and aggravated diffuse abdominal pains for three days. His personal history was unremarkable except for the hypertension of one month duration. Physical examination revealed a palpable mass-like lesion in right lower quadrant. The results of other laboratory investigations, including routine blood tests, liver tests, urine analysis and serum tumor marker (carcinoembryonic antigen) were within normal limits, except for the mild anemia (hemoglobin, 8.7 g/dL). The MDCT of the abdomen was performed for further evaluations on a palpable abdominal mass, including unenhanced CT scans and dynamic contrast-enhanced CT scans with a non-ionic contrast medium (Omnipaque 350, GE Healthcare, Oslo, Norway). The volume of 100 mL contrast medium was injected at a rate of 3 mL/s via an antecubital vein. The dynamic contrast-enhanced abdominal MDCT scan demonstrated a well-defined, round-shaped, exophytic growing mass with central necrosis, which measured 6 cm in greatest dimension of the mid jejunum. The lesion showed poor contrast enhancements. In addition, significant dilatation of proximal jejunum with abrupt beaking appearances suggestive of tethering adjacent the mass was demonstrated (Fig. 1). We presumed the small bowel obstruction caused by tumor seeding adjacent proximal jejunum. In this patient, the jejunal loops were located in the lower right quadrant area, probably due to counterclockwise twist of the small bowel mesentery associated with the tethering adjacent the mass. There was no significant lymphadenopathy. Considering the abovementioned CT features, the patient was diagnosed as adenocarcinoma in jejunum. Because the adenocarcinoma is the most common type of primary small intestinal malignancy, the epithelial origin tumor can lead to early bowel obstructions as compared with mesenchymal origin tumor. Our differential diagnosis was mesenchymal origin tumor such as gastrointestinal stromal tumor (GIST) or lymphoma. A mass with central necrosis is an overlapping CT appearance of many small bowel neoplasms, and especially the mesenchymal origin tumors tend to grow exophytically and rarely cause bowel obstructions even though the tumor size is relatively large. In this case, bowel obstruction was presumed due to seeding nodules rather than the mass itself. Malignant carcinoid tumor and leiomyosarcoma were also considered as a differential diagnosis, even though they are rare, small intestinal mesenchymal tumors. Thus, they may appear as a large intraluminal ulcerating lesion.
Exploratory laparotomy revealed a mass of small bowel located in jejunum, about 45 cm apart from the duodenojejunal junction with invasion into the serosal surface and the mesenteric fat. A more proximal segment of jejunum was distorted without the definite palpable mass. A portion of jejunal segment along with adherent loop of proximal jejunum was resected.
Segmental resection revealed a mass located at the jejunum. The resected specimen showed a large, irregular-shaped, fungating mass with friable and necrotic surface, measuring 4 × 5 cm (Fig. 2). The tumor extended to the subserosa and focally infiltrated the serosa of the adherent, small bowel loop, macroscopically.
Microscopically, the tumor consisted mostly of discohesive polygonal giant cells with a few spindle cells. These cells were arranged in sheets or haphazardly. Giant cells have dense eosinophilic cytoplasm and pleomorphic nuclei. On immunohistochemistry, tumor cells showed diffused strong positive immunoreactivities for vimentin and focal strong positive reactions for cytokeratin (Fig. 3). The immunoreactivities were negative staining against the other antibodies, such as CD20, CD10 and c-kit. Although carcinomatous component was not recognized at microscopic examination, the carcinomatous nature of the tumor was evident after immunohistochemistry examination, cytokeratin suggestive finding of carcinomatous nature of tumor was positive. The histopathological and immunohistochemical transitions between the adenocarcinoma area and the spindle cell area suggested that the sarcomatous elements originated from the adenocarcinoma during tumor progressions. Therefore, the final diagnosis was sarcomatoid carcinoma. Additional treatment was not given and the patient was lost for follow-ups.
Sarcomatoid carcinomas are rare tumors characterized by a mixture of carcinomatous and sarcomatous features. Variable synonyms including carcinosarcoma, metaplastic carcinoma, spindle cell carcinoma, and pleomorphic carcinoma have been used.
Histologically, sarcomatoid carcinomas can be classified into biphasic or monophasic (6). Biphasic tumors are composed of epithelial-looking and mesenchymal-like cells. Monophasic tumors show a predominance of the mesenchymal-like component, with minimal to absent epitheloid area. Because monophagic sarcomatoid carcinoma can be confused with other sarcomas and minimal epitheloid component can be missed, a wide-range panel of immunohistochemical biomarkers should be performed for the differential diagnosis. Most of the tumors show positivity for cytokeratin and vimentin (6). In this case, cytokeratin and vimentin were positive and the carcinomatous nature of the tumor was evident only after the immunohistochemistry test.
Clinically, sarcomatoid carcinomas in the small intestine primarily affected middle-aged to older patients, with a mean age of 57 years. The male to female ratio was 1.5 : 1 (6). Symptoms may include abdominal pain, intestinal obstruction, palpable abdominal mass, gastrointestinal bleeding, and anemia (5, 8). Sarcomatoid carcinomas in the lower intestinal tract have aggressive clinical courses, often presented with symptoms or signs related to distant metastasis. On the other hand, sarcomatoid carcinomas in upper aerodigestive tract including the esophagus and stomach have polypoid growth patterns and can be early diagnosed in their course, and accordingly, are associated with relatively favorable prognosis. In this case, the patient presented with acute abdominal pain and palpable mass was detected through physical examination. We presumed that acute symptom was related to the bowel obstruction which was caused by the tumor seeding. Although laboratory finding revealed mild anemia, the association between anemia and the lesion was unclear in this case.
Small intestinal sarcomatoid carcinomas arise most frequently in the jejunum and ileum (2-9). Majority of sarcomatoid carcinomas in the small intestines showed polypoid or ulcerofungating mass with or without central ulceration, and are frequently necrotic and hemorrhagic, with an average of 7 cm in greatest dimension (range, 3-16 cm) (Table 1) (2-9).
CT findings of small intestinal sarcomatoid carcinoma were described in a few reported cases (Table 1) (1-10). Symmetric or asymmetric wall thickening with fold effacement was noted in three cases (3, 4, 7). CT failed to detect the lesion in three cases (6, 8, 9). Paik and Choi (10) reported duodenal sarcomatoid carcinoma mimicking focal pancreatic head swelling. Most of the reported cases were not applicable radiologically. In the present case, CT scan showed a predominantly exophytic mass with central necrosis. This imaging finding is consistent with the gross morphology noted here (Fig. 2) and in previous reports (2-10). It is remarkable how similar the gross specimen is to the CT finding of the mass when compared with the previous reports. However, this CT finding is remarkably similar to that of the mesenchymal tumor such as GIST, lymphoma, carcinoid or leiomyosarcoma. So, it is not easy to distinguish sarcomatoid carcinoma from mesenchymal tumors radiologically. We presume that it is because monophasic sarcomatoid carcinoma includes minimal epithelial component. Further studies for comparing CT finding of biphasic and monophasic sarcomatoid carcinoma will be necessary. Small bowel series was done together with the CT in several cases (6-8, 10). The study revealed the intraluminal polypoid mass (6, 8, 10), although CT failed to detect the lesion (6, 8) or mimicked the pancreatic cancer (10). Thus, it meant that diverse diagnostic tools may be necessary to detect or diagnose the small bowel lesion correctly.
Evaluating primary small intestinal tumors are not easy due to its acceptability. Although immunohistochemistry plays a vital role in the correct diagnosis, CT scans can be one of the essential diagnostic modalities. Sarcomatoid carcinomas were rarely reported in the small intestine, and there were few reported cases which concerned radiologic features and should be differentiated. Therefore, data collection on radiologic findings of sarcomatoid carcinomas is important.
Surgical resection remains the mainstay of treatment, as patients show poor responses to chemotherapy and radiation treatment (6). Small intestinal sarcomatoid carcinomas show aggressive behaviors and have a poor prognosis. The duration of survival is generally only a few months to three years of diagnosis (6, 8). The histological features, tumor stage, and outcome of the reported cases indicate that this neoplasm generally persues a highly aggressive and malignant biological course with rapid growth and wide local infiltration which leads to a poor prognosis. The best predictors of outcome in sarcomatoid carcinoma seem to be tumor location, size, invasion depth, and the clinical stage of the disease. Radical surgery with adjuvant chemotheraphy, and close follow-up are necessary for the management of this disease.
In conclusion, we present a rare case of sarcomatoid carcinoma in the small intestine, composed of mixed malignant epithelial and mesenchymal cells, with only 26 cases reported to date. In this case, the CT showed ulcero-fungating mass with necrosis, which corresponds to the gross morphology. Although it is a nonspecific finding, the sarcomatoid carcinoma must be kept in mind for the differential diagnosis of small intestinal malignancies due to its aggressive nature and poor prognosis.
Figures and Tables
Fig. 1
CT images in a 70-year-old man with abdominal pain. Axial pre-contrast (A) and delayed phase (B) images show a well-defined, round shaped fungating mass (solid arrow) with central necrosis. Coronal reconstruction images on delayed phase CT scan (C, D) show focal wall thickening suggesting tumor seeding (open arrow in C) and beaking appearance (curved arrow in D) with proximal bowel dilatation.

Fig. 2
Photograph of the surgical specimen. The gross specimen shows a fungating necrotic mass in the jejunum.
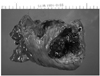
Fig. 3
Pathologic findings of the mass [A. H&E (× 100). B. H&E (× 400). C. Cytokeratin (× 200). D. Vimentin (× 200)]. Microscopically, the tumor consisted mostly of discohesive polygonal giant cells with a few spindle cells (A, B). Tumor cells showed focal strong staining for cytokeratin (C) and diffuse strong staining for vimentin (D).

References
1. Iezzoni JC, Mills SE. Sarcomatoid carcinomas (carcinosarcomas) of the gastrointestinal tract: a review. Semin Diagn Pathol. 1993; 10:176–187.
2. Robey-Cafferty SS, Silva EG, Cleary KR. Anaplastic and sarcomatoid carcinoma of the small intestine: a clinicopathologic study. Hum Pathol. 1989; 20:858–863.
3. Padma SK, Permi HS, Patil C, Mathias M. Aggressive monophasic sarcomatoid carcinoma of small intestine - a rare case report with review of literature. NUJHS. 2012; 2:45–47.
4. Agrawal S, Trivedi MH, Lukens FJ, Moon C, Ingram EA, Barthel JS. Anaplastic and sarcomatoid carcinoma of the small intestine: an unusual tumor. J Clin Gastroenterol. 1999; 29:99–101.
5. Yucel AF, Kocakusak A, Arikan S, Demirbag N, Tarlaci A, Batur S. A rare cause of acute abdomen: perforated primary sarcomatoid carcinoma of the small intestine - report of a case, with a brief review of the literature. J Cancer Res Ther. 2011; 7:348–350.
6. Reid-Nicholson M, Idrees M, Perino G, Hytiroglou P. Sarcomatoid carcinoma of the small intestine: a case report and review of the literature. Arch Pathol Lab Med. 2004; 128:918–921.
7. Lee SE, Park SY. Sarcomatoid carcinoma of the small intestine: a rare and highly aggressive tumor. J Korean Surg Soc. 2012; 83:321–324.
8. Moriwaki Y, Sugiyama M. Severe anemia inducing preshock caused by sarcomatoid carcinoma of the small intestine. Int Surg. 2009; 94:164–170.
9. Tsukadaira A, Koizumi T, Okubo Y, Takashi S, Koide N, Arai K, et al. Small-intestinal sarcomatoid carcinoma with superior vena cava syndrome. J Gastroenterol. 2002; 37:471–475.
10. Paik HJ, Choi YM. A case of carcinosarcoma in duodenum. J Korean Surg Soc. 1991; 41:549–553.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download