Abstract
Congenital anomalies involving the coronary sinus (CS) tend to receive relatively little attention because they rarely cause clinical symptoms or disturbances of cardiac function. However, as imaging modalities have been developed over time, the detailed anatomy of the heart, including CS anomalies, can now be evaluated more precisely. The purpose of this pictorial review is to illustrate multi-detector computed tomography findings of various congenital anomalies of the CS. The cardiac venous system and its embryologic development are also described in detail to familiarize radiologists with various congenital anomalies of the CS.
As imaging modalities evolve, the detailed anatomy of the heart, including congenital anomalies, can be detected more easily. However, even with such advancements, anomalies involving the coronary sinus (CS) have received relatively little attention, perhaps because they do not usually cause clinical symptoms or functional disturbances.
However, precise diagnosis of anatomic variants of the CS and its tributaries is crucial to correct the performances of various interventional procedures, such as cardiac resynchronization therapy and percutaneous transvenous mitral annuloplasty (1-3). Additionally, cardiac surgeons must be aware of the anatomy of the CS, as it is used as a route for injecting cardioplegic solutions to minimize cardiac activity and to allow the operation to be performed in a still and bloodless field. This process also allows surgeons to reduce the aortic cross-clamping times and avoid interruptions. Retrograde cardioplegia utilizes the uniform CS venous system, because it has a more uniform venous network and is not affected by coronary artery diseases (3). In patients with severe coronary artery diseases, antegrade cardioplegia may fail to supply the cardioplegic solution to the myocardium sufficiently due to stenotic or occluded arteries. Thus, in such situations, retrograde cardioplegia is preferred. However, failure to recognize congenital anomalies of the CS, such as atresia of the CS orifice or agenesis of the CS, may lead to difficult or failed retrograde coronary venous catheter insertions.
Congenital anomalies involving the CS can be classified into four groups based on the following criteria (4, 5): 1) enlargement of the CS with or without a left-to-right shunt, 2) absence of the CS, 3) atresia of the right atrial ostium of the CS, and 4)hypoplasia of the CS. These anomalies are considered to be the faulty embryologic development results from the heart.
The purpose of this pictorial review is to illustrate and classify CS congenital anomalies, and to present several incidentally founded cases. The cardiac venous system and its embryologic development are also being described in detail to familiarize radiologists with various anomalies.
A recent anatomic classification divides the cardiac veins into two main groups: tributaries of the greater cardiac venous system (CVS), and, tributaries of the lesser CVS.
The greater CVS is divided into two subgroups: CS tributaries and non-CS tributaries. The CS tributaries include the CS as the major component of the CVS and collectively drains about 75% of the coronary venous blood (3). The CS is approximately 45 mm in length and 10-20 mm in diameter. The CS lies along the posterior atrioventricular groove and empties into the right atrium through the orifice guarded by the thebesian valve.
The first branch of the CS is the posterior interventricular vein (or the middle cardiac vein), which courses in the posterior interventricular groove. The next main branches are the posterior vein of the left ventricle and the left marginal vein. The CS continues as the great cardiac vein, which courses in the left atrioventricular groove (6). The anatomic landmark of the junction between the CS and the great cardiac vein junction is the orifice of the oblique vein of Marshall (the oblique vein of the left atrium), the valve of Vieussens, or the left margin of the myocardial sleeve of the CS. On cardiac CT imaging, the valve of Vieussens can be identified as an annular narrowing on the external surface (Fig. 1) (1). Among these tributaries, the left marginal vein and the posterior vein of the left ventricle are typically used for cardiac resynchronization therapy (7), and thus, the variability of these veins should be assessed with imaging prior to therapy.
Normal venous development is a complex process of progression and regression (Fig. 2) (1-5, 8, 9). During the fourth week of embryonic development, three paired veins drain into the tubular heart, namely the vitelline, umbilical, and common cardinal veins. The opening of the sinus venosus into the primordial atrium is large at first and gradually moves to the right side. The right umbilical vein and the left vitelline vein both regress during the fifth week. By the tenth week, the left common cardinal vein is occluded, and the left horn of the sinus venosus forms the CS and the oblique vein of Marshall (oblique vein of left atrium) (9). The right horn of the sinus venosus is gradually combined with the right atrium and forms the posterior wall of the right atrium. A connecting vessel between the right and left anterior cardinal veins becomes the left brachiocephalic vein and shunts blood from the left to the right side.
Congenital anomalies of the CS were classified into four types by Mantini et al. (5) in 1966 (Table 1, Fig. 3).
Enlargement of the CS may result from many acquired causes, including ventricular systole of the cardiac phase, heart failure, cardiomyopathy, or atrial fibrillation (1). Meanwhile, increased blood flow volume through anomalous congenital communications also causes enlargement of the CS. Such anomalies can be divided into two groups, depending on the existence of a left-to-right shunt into the CS.
When the CS receives anomalous systemic venous return, it becomes enlarged. Possible anatomic variations in this subgroup include a) persistent left superior vena cava (PLSVC) confluent with the CS, b) partial anomalous hepatic venous return to the CS, and c) continuity of the inferior vena cava with the left superior vena cava through the hemiazygos vein.
PLSVC (Fig. 3A) is the most common thoracic venous anomaly. The prevalence of PLSVC is reported to be 0.3% in healthy individuals and 4.4% in patients with congenital heart disease (10). This anomaly results from persistent patency of the left anterior cardinal vein, which normally obliterates during development. A PLSVC usually drains into the right atrium through a dilated CS (Figs. 4, 5); however, it may also drain directly into the right atrium in some cases.
This anomaly usually occurs as an isolated lesion, but it can be associated with other congenital cardiac lesions, including the PLSVC draining into the left atrium, interatrial septal defect, or single atrium. The absence of the right superior vena cava also accounts for approximately 20% of PLSVC (Fig. 6) (11). In this case, both the left and right brachiocephalic veins drain into the PLSVC.
An anomalous venous channel arising from the liver pierces the diaphragm and the pericardium. It then passes through the posterior aspect of the heart to join the CS (Fig. 3B).
In this condition, instead of connecting to the hepatic segment, the suprarenal segment of the developing inferior vena cava joins the hemiazygos vein, which in return joins a PLSVC. The PLSVC further drains into the CS, resulting in enlargement (Fig. 3C). This condition may also be associated with other cardiac anomalies, including abnormal positioning of the heart, partial inversion of the abdominal viscera, or polysplenia (5, 9).
The CS may also become enlarged when oxygenated blood shunts through anomalous communications. These communications can be divided into two groups: low- and high-pressure, left-to-right shunts.
This communication may occur either indirectly or directly. In cases of indirect communication, an anomalous vein passes over the lateral wall of the left atrium, and connects the CS with the left atrium. Embryologically, this anomalous vein is considered to be a persistent vessel connecting the left atrium or pulmonary vein to the cardinal venous system in response to a partially obstructed CS ostium (12). Blood flow in this channel is expected to be in a left-to-right direction under physiological pressure.
Unroofed CS is a rare anomaly in which communication between the CS and the left atrium is formed directly as a result of the partial or complete absence of the CS roof (Figs. 3D, 7, 8). This entity is the rarest type of atrial septal defect and is often associated with PLSVC (4, 5, 9). This condition usually occurs with a left-to-right shunt; however, if the PLSVC is connected to the CS, systemic venous blood may be diverted to the left atrium via the fenestration. This diversion may result in a right-to-left shunt, which can cause cerebral emboli or brain abscesses.
This anomaly is either total or subtotal forms, but total anomalous pulmonary venous connection to the CS is the more common form. Under this condition, each of the pulmonary veins forms a pulmonary venous confluence that connects the CS.
Coronary artery-coronary sinus fistula is a rare coronary arteriovenous fistula. This condition leads to a high-pressure, left-to-right shunt into the CS that is usually dilated. The involved artery becomes elongated and tortuous, and an aneurysm may sometimes form (5).
This condition is usually accompanied by other anomalies, such as a PLSVC connected to the left atrium or an atrial septal defect. There are three subdivisions based on the type of atrial septal defect and the existence of additional anomalies (4, 5).
This atrial septal defect is located in the posteroinferior angle of the atrial septum that is normally occupied by the CS (Fig. 3E). This condition is considered to arise from the developmental failures of wall formation between the CS and the left atrium. Left-to-right interatrial shunts occur through defect in the left atrial side, which is continuous with the orifice of the CS opening on the right atrial side of the septum. However, when the right atrial pressure exceeds the left atrial pressure, a right-to-left shunt can occur and allows paradoxical emboli to enter the systemic arterial circulation.
The persistent common atrioventricular canal shows a more extensive malformation of the atrioventricular valves in the form of defects of the atrial septum and the persistent common atrioventricular canal (Fig. 3F). Congenital cardiac disease with asplenia is another syndrome that includes the absence of the CS and splenic agenesis.
In this condition, the CS lies in normal position, but has a blind ostium to the right atrium (Figs. 9, 10). If accompanied by a PLSVC, it carries blood from the CS to the right atrium through the left innominate vein. The condition may occur as an isolated anomaly, although it can be associated with other cardiac malformations (1, 4, 5).
Blood from the CS passes the left superior vena cava in a retrograde direction, then moves into the right superior vena cava through the left innominate vein and eventually into the right atrium (Fig. 3G). In this circumstance, the PLSVC is the only outflow channel for the CS. Procedures that interrupt coronary venous drainage, such as ligation of the PLSVC, can lead to myocardial ischemia or coronary venous hypertension (2).
This anomaly has a narrow left superior vena cava joined to the CS with the CS communicating with the left atrium, and is somewhat similar to the unroofed CS mentioned previously (Fig. 3H). The blood flows from the CS through this opening into the left atrium.
In this rare subtype, some cardiac veins may not join the CS and may individually empty the atrial chambers through dilated thebesian channels due to a failure when joining the coronary sinus (1, 5). Unlike atresia or stenosis of the CS orifice (Fig. 11) which is a focally involved lesion at the CS orifice; hypoplasia and agenesis involve lesions throughout the CS diffusions.
Congenital anomalies involving the CS can be classified into four groups and several subgroups depending on the presence of additional anomalies. These defects do not usually cause significant symptoms or dysfunctions; however, during interventional treatment for heart disease or retrograde cardioplegia during cardiac surgery, knowledge about the anatomy and the potential anomalies of the CS is important. Recognizing variations of congenital CS anomalies may help avoid errors during these procedures.
Figures and Tables
Fig. 1
Venous drainage of the heart. Anterior (A, C) and posteroinferior (B, D) views of the heart. The coronary sinus (CS) runs along the inferior aspect of the heart in the atrioventricular groove before emptying into the right atrium (RA). The posterior interventricular vein (PIV) courses in the posterior interventricular groove from the base to the apex. The posterior vein (PV) of the left ventricle and the left marginal vein (LMV) are the next main branches. The great cardiac vein (GCV) courses in the left atrioventricular groove with the left circumflex artery. The anterior interventricular vein (AIV) continues in the anterior interventricular groove, adjacent to the left anterior descending artery.
Note.-★= valve of Vieussens (junction between CS and GCV), LA = left atrium, LV = left ventricle, RV = right ventricle
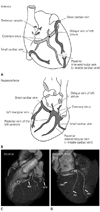
Fig. 2
Development of the cardiac veins. Posterior view of the heart of an embryo at four weeks (A), eight weeks (B), and ten weeks (C). By the eighth week, the umbilical veins and the left vitelline vein regress. The left and right anterior cardinal veins connect through a vessel that becomes the left brachiocephalic vein. The right horn of the sinus venosus becomes the posterior wall of the right atrium, and the cardinal veins become the vena cava. The left common cardinal vein is occluded, and the left horn of the sinus venosus forms the coronary sinus and the oblique vein of the left atrium (vein of Marshall). Adapted from Moore KL, Persaud TVN. The developing human: clinically oriented embryology, 7th ed (8).
Note.-LA = left atrium, RA = right atrium

Fig. 3
Illustrations of congenital anomalies of the coronary sinus (CS). Enlargement of the CS associated with (A) persistent left superior vena cava (PLSVC), (B) partial anomalous hepatic venous connection, (C) continuity of the inferior vena cava with PLSVC through the hemiazygos vein, and (D) the absence of the CS roof (unroofed CS). The absence of the CS associated with (E) PLSVC and atrial septal defect. (F) PLSVC and a variety of persistent common atrioventricular canals. The atrial septal defect involves the entire lowermost portion. Atresia of the right atrial ostium of the CS associated with (G) functional PLSVC, with blood returning in a retrograde direction into the right atrium through the left innominate vein; (H) a narrow PLSVC joins an anomalous CS that communicates with the left atrium; and (I) multiple communications between CS and related atria. Adapted from Mantini E, Grondin CM, Lillehei CW, Edwards JE. Circulation 1966;33:317-327 (5).

Fig. 4
Incidentally found persistent left superior vena cava (PLSVC) with a dilated coronary sinus (CS) in a 49-year-old female.
A. Axial CT MIP image showing that the PLSVC (arrow) lies lateral to the left main pulmonary artery. The right superior vena cava is noted in its normal position.
B. The dilated CS (arrows) drains into the right atrium (RA).
C. The parasternal long axis view in transthoracic echocardiography showing an abnormally dilated coronary sinus (arrow).
Note.-LA = left atrium, LV = left ventricle, MIP = maximum intensity projection

Fig. 5
Incidentally found persistent left superior vena cava (PLSVC) in a 17-year-old boy who underwent both central venous catheter and chest tube insertion due to a traffic accident.
A. Plain chest radiograph showing the hemodialysis catheter in the right superior vena cava and the subclavian catheter in the PLSVC.
B. Left superior venacavogram confirming that the persistent left superior vena cava drains through the coronary sinus into the right atrium.
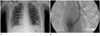
Fig. 6
Absence of the right superior vena cava with persistent left superior vena cava (PLSVC).
A. Axial CT image showing the PLSVC (arrow) without the right superior vena cava.
B. The severely dilated coronary sinus (CS) (arrows) drains into the right atrium (RA).
C, D. 3D VR image (C) and curved MPR image (D) showing the PLSVC draining into the severely dilated CS (arrows) inferior to the left atrioventricular groove.
Note.-3D VR = three dimensional volume rendering
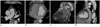
Fig. 7
Incidental unusual communication of the severely dilated great cardiac vein with the left atrium and a partially unroofed coronary sinus (CS) in a 69-year-old female.
A. Axial CT MIP images demonstrate a severely dilated great cardiac vein (white arrow) having a communication with the left atrium just below the left superior pulmonary vein level.
B. The coronary sinus (white arrows) is also dilated and drains into the right atrium.
C. Curved MPR image showing an abnormal communication of the coronary sinus with the left atrium (white arrows). The partially unroofed coronary sinus can be seen (black arrow).
Note.-MIP = maximum intensity projection, MPR = multiplanar reconstruction, RA = right atrium, LA = left atrium
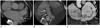
Fig. 8
Incidentally found partially unroofed coronary sinus (CS) in a 65-year-old woman.
A. Curved MPR image showing a partially unroofed CS, the site of unroofing (arrows), and dense contrast (left-to-right shunt) entering the right atrium (RA).
B. 3D VR image showing a partial direct communication (arrows) between the CS and the left atrium and mild dilatation of the CS.
Note.-MPR = multiplanar reconstruction, 3D VR = three dimensional volume rendering
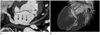
Fig. 9
A case of coronary sinus (CS) orifice atresia with a persistent left superior vena cava (PLSVC) and an aberrant communicating vein into the right atrium in a 48-year-old male. (A) Left lateral view of the 3D VR image showing the PLSVC communicating with a mildly dilated CS. 3D VR images (B, C) and curved MPR image (D) showing the CS terminating as a blind sac without any grossly visible communication with the right atrium (RA). There is also an unusual exit (arrows) from the CS through the posterior interventricular vein that runs into the right ventricular and atrial wall. This unusual vein communicates with the RA cavity via a small orifice.
Note.-LA = left atrium, MPR = multiplanar reconstruction, RV = right ventricle, 3D VR = three dimensional volume rendering

Fig. 10
Coronary sinus (CS) orifice atresia with persistent left superior vena cava (PLSVC) in a 73-year-old female.
A. Axial CT MIP image showing a blind ostium (star) of the CS to the right atrium.
B, C. 3D VR images showing (B) a dilated CS (arrows) and (C) the PLSVC (arrows) communicating with the dilated CS.
Note.-MIP = maximum intensity projection, 3D VR = three dimensional volume rendering
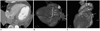
Fig. 11
Coronary sinus (CS) orifice stenosis with aberrant coronary venous drainage into the left atrium (LA) in a 59-year-old female. CT MIP images showing (A) the CS orifice stenosis (white arrow) and (B) aberrant coronary venous drainage (white arrows) into the LA. 3D VR image (C) also showing the CS orifice stenosis (black arrow) and aberrant coronary venous drainage into the LA (white arrows).
Note.-MIP = maximum intensity projection, 3D VR = three dimensional volume rendering

References
1. Saremi F, Muresian H, Sánchez-Quintana D. Coronary veins: comprehensive CT-anatomic classification and review of variants and clinical implications. Radiographics. 2012; 32:E1–E32.
2. Muster AJ, Naheed ZJ, Backer CL, Mavroudis C. Is surgical ligation of an accessory left superior vena cava always safe? Pediatr Cardiol. 1998; 19:352–354.
3. Ruengsakulrach P, Buxton BF. Anatomic and hemodynamic considerations influencing the efficiency of retrograde cardioplegia. Ann Thorac Surg. 2001; 71:1389–1395.
4. Chou MC, Wu MT, Chen CH, Lee MH, Tzeng WS. Multidetector CT findings of a congenital coronary sinus anomaly: a report of two cases. Korean J Radiol. 2008; 9:Suppl. S1–S6.
5. Mantini E, Grondin CM, Lillehei CW, Edwards JE. Congenital anomalies involving the coronary sinus. Circulation. 1966; 33:317–327.
6. O'Brien JP, Srichai MB, Hecht EM, Kim DC, Jacobs JE. Anatomy of the heart at multidetector CT: what the radiologist needs to know. Radiographics. 2007; 27:1569–1582.
7. Bax JJ, Abraham T, Barold SS, Breithardt OA, Fung JW, Garrigue S, et al. Cardiac resynchronization therapy: Part 2--issues during and after device implantation and unresolved questions. J Am Coll Cardiol. 2005; 46:2168–2182.
8. Moore KL, Persaud TVN. The developing human: clinically oriented embryology. 7th ed. Philadelphia, PA: Saunders;2003. p. xv. p. 560.
9. Anderson RH, Brown NA, Moorman AF. Development and structures of the venous pole of the heart. Dev Dyn. 2006; 235:2–9.
10. Cha EM, Khoury GH. Persistent left superior vena cava. Radiologic and clinical significance. Radiology. 1972; 103:375–381.
11. Pálinkás A, Nagy E, Forster T, Morvai Z, Nagy E, Varga A. A case of absent right and persistent left superior vena cava. Cardiovasc Ultrasound. 2006; 4:6.
12. Edwards JE, DuShane JW, Alcott DL, Burchell HB. Thoracic venous anomalies. III. Atresia of the common pulmonary vein, the pulmonary veins draining wholly into the superior vena cava. AMA Arch Pathol. 1951; 51:446–460.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download