Abstract
Epithelial-myoepithelial carcinoma (EMC) is a rare tumor that commonly involves the salivary glands. EMC arising from the nasal cavity is one of the most unusual cases. We describe a case of a 48-year-old patient who is presented with bilateral nasal obstruction for several months. Multidetector computed tomography reveals expansile, well-defined, heterogeneous enhancing soft tissue masses filling the nasal cavity with bony destruction of hard palate and maxillary alveolar ridge. The carcinoma was histologically characterized by a mixture of trabecular structure with myoepithelial cells and ductal cells, which are confirmed by electron microscopy and immunohistochemistry.
Epithelial-myoepithelial carcinoma (EMC) usually occurs in the parotid gland, representing about less than 1% of all salivary gland tumors (1, 2). In addition, the major sites of involvement are the maxillary sinus, trachea, larynx, hypopharynx, and minor salivary glands, breast and lung, although it has also been reported in the mucoserous glands of the upper and lower aerodigestive tracts (1, 2). EMC originating from the nasal cavity is very rare. The low-grade malignant epithelial neoplasm is composed of variable proportions of ductular cells with large, clear staining, myoepithelial cells arranged around the periphery of the ducts (2).
On multidetector computed tomography (MDCT), the majority of EMC shows inhomogeneous enhancement patterns on previously reported cases (3-6). The diagnosis of the EMC is difficult for the nonspecific radiological findings.
We report a 48-year-old woman with epithelial-myoepithelial carcinoma arising from the nasal cavity with bony destruction.
A 48-year-old woman presented bilateral nasal obstruction for several months. On examination, a firm mass, filling the right nasal cavity and displacing the septum to the left side, was observed. No lymphadenopathy was presented. The general physical examinations were within normal limits. A multidetector 64-channel computed tomography (Aquillion 64; Toshiba Medical Systems, Tokyo, Japan with the following parameters: a section thickness of 0.2 mm, a tube current of 250 mA, a tube potential of 120 kV) scan of the paranasal sinus was performed. The computed tomography (CT) images had two phases: non-contrast phases and enhanced phases. Enhanced phase was performed with automatic bolus tracking techniques after injection of nonionic contrast material (iohexol, Omnipaque; GE Healthcare, Milwaukee, WI, USA). The CT number (in Hounsfield units, HU) of the tumor was measured by means of a circular region of interest (ROI). The ROI circle of the tumor was made as large as possible within the tumor. MDCT showed 50 × 45 × 37 mm size well-defined soft tissue mass filling the nasal cavity (Fig. 1). Mass extended into nasopharyngeal space posteriorly and right maxillary alveolar ridge anteriorly on sagittal CT scans. There was also bony destruction of hard palate and maxillary alveolar ridge due to tumor invasions. An enhanced phase axial CT showed heterogeneous enhancement (145 HU) of the tumor with septum-like high attenuation portions. The same lesion was detected 50 HU on non-contrast CT scan. There were also multiple non-enhancing low attenuation lesions in tumor. Under general anesthesia, the patient underwent tumor resection. The bony destruction portion was not removed. The cut surface of the mass showed a lace-like appearance with multiple whitish portions in a myxoid background. A microscopic examination revealed that the tumor was composed of epithelial and myoepithelial differentiation and increased proliferative activities.
The microscopic findings showed duct-like structures by hematoxylin-eosin staining with an inner layer of epithelial cells and outer layers of clear cells (Fig. 1D). Immunohistochemical findings of the tumor showed expression of cytokeratin, p63 and ki67 (Fig. 1E, F), and the pathologic diagnosis was epithelial-myoepithelial carcinoma.
EMCs are rare tumors of the salivary glands and represents 0.5-1% of all salivary gland neoplasms. The majority of these are seen in the parotid gland (1, 2). There are cases reported arising from submandibular gland and minor salivary glands located in various sites but only 9 cases are reported from nasal cavity in the English literature (3-5, 7-12). Theoretically, they can arise in any organ where a double layer duct pattern exists such as lung, kidney, pancreas, uterus and ovary, and are considered to be a variant of clear cell carcinoma. These tumors differ by exhibiting greater epithelial cellularity with less stroma as compared to pleomorphic adenomas in the salivary glands (13). This histological characteristic explains the difficulty in differential diagnosis of EMCs from pleomorphic adenomas in the nasal cavity.
These tumors are thought to be of low-grade malignancy, although a significant number of cases have been reported with local regional recurrence and distant metastasis (8). Although the index patient presented was pathologically confirmed as low-grade salivary gland type neoplasm, the tumor located in the nasal cavity invaded into the adjacent bony structure. The initial tumor image on CT scan showed destruction of adjacent hard palate and maxillary alveolar ridge. The tumor also showed an unusual large size. These features of tumor may be considered as the aggressive form of carcinoma.
Histopathologically, most EMCs show a distinguished nodular or multinodular growth pattern and classic biphasic tubular histology of inner ductal cells with cuboidal epithelium and outer clear myoepithelial cell layers (1, 2). The inner cells of these ductules constitute the epithelial component of EMCs. The outer cell layer is the clear cell myoepithelial component of EMCs. The microscopic findings of our case showed tumor was in the form of glands that were lined by 2 cell types, the inner epithelial and the outer myoepithelialcells. The immunohistochemical staining pattern was the inner cuboidalcells which did not show immunoreactivity for myoepithelial markers such as p63, Ki67 and smooth-muscle antigen (Fig. 1E, F) but exhibited positivity with cytokeratin and epithelial membrane antigen. In contrast, the outer layer of cuboidal cells was positive p63 and Ki67 immunostain (Fig. 1E, F), confirming that they were myoepithelial.
Many biological factors influence the enhancement patterns of tumor. Vascularity, histopathological cell types, and histological components of the tumors are important keys for enhancement (14). A benign myoepithelioma of the spindle cell type with areas of hyalinization and a myoepithelioma of the plasmacytoid cell type with myxoid stroma shows faint enhancement. On the other hand, the myoepithelioma of the spindle cell type with fibrous stroma detected with intense enhancement (6). This suggests that the histological component, the stroma, may affect the enhancement pattern of the tumor (6). The tumor of our case had multiple, loculated, non-enhancing portion and septum-like, intensely enhanced portion. On biological view, the non-enhancing or faint enhancing portions corresponded to the spindle cell type of myxoid stroma component and the enhancing portions are to the fibrous stroma component. The portion of the tumor into the hard palate and right maxillary alveolar ridge also showed intense enhancement (140-150 HU). Although the portion with bony destruction was not pathologically confirmed, intensely enhanced pattern suggests fibrous stroma component of the tumor.
The etiology of these tumors is not yet clearly known because of the limited number of cases. These tumors are considered as low-grade malignant but EMC has an unusual aggressive feature (9). The index patient did not show any regional recurrences or distant metastasis of follow-ups yet, but long-term follow-ups are needed. The prognostic factors of these tumors are not well-defined. However, tumor size, lymph node involvement, distant metastasis, and frequent mitotic figures are associated with prognosis.
In conclusion, the CT findings of a case of EMC of the nasal cavity gland are presented. Because the EMC is rare tumor and the imaging findings of the EMC appear to be non-specific, the initial role of the radiologist is the preoperative identification and localization of the mass. The differential diagnosis of the EMC could be adenomyoepithelioma, adenoma, adenocarcinoma, clear cell tumor, and so on. However, once the diagnosis was being made, careful periodic postoperative imaging is needed for detecting the potentially local recurrence.
Figures and Tables
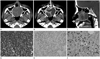 | Fig. 1A 48-year-old woman with epithelial-myoepithelial tumor in nasal cavity.
A. An expansile well-marginated soft tissue mass, filling the nasal cavity, shows heterogenous nature on non-enhancing axial CT scan (arrows).
B. On enhanced axial CT scan shows multiloculated low attenuation lesions (thin arrows) and septum like enhancing portion in mass (thick arrow).
C. Mass extends into nasopharyngeal space posteriorly (thin arrow) and there was also bony destruction of hard palate and maxillary alveolar ridge due to tumor invasion on sagittal CT sacn (thick arrows).
D. Each tubules are lined by two layers of cells, inner eosinophilic epithelial cells and outer myoepithelial cells (hematoxylin-eosin, × 400).
E. Immunohistochemical analysis shows that the expression of p63 (original magnification, × 400).
F. Immunohistochemical analysis shows that the expression of Ki67 (original magnification, × 400).
|
References
1. Ellis GI, Auclair PL. Tumors of the Salivary Glands. In : Rosai J, editor. Malignant epithelial tumors. Washington, DC: Armed Forces Institute of Pathology;1996. p. 268–289.
2. Batsakis JG, el-Naggar AK, Luna MA. Epithelial-myoepithelial carcinoma of salivary glands. Ann Otol Rhinol Laryngol. 1992; 101:540–542.
3. Lee HM, Kim AR, Lee SH. Epithelial-myoepithelial carcinoma of the nasal cavity. Eur Arch Otorhinolaryngol. 2000; 257:376–378.
4. M'sakni I, Laabidi B, Bougrine F, Sabbegh-Znaïdi N, Benzarti S, Chebbi K, et al. [Epithelial-myoepithelial carcinoma of the nasal cavity]. Ann Otolaryngol Chir Cervicofac. 2007; 124:228–231.
5. Yamanegi K, Uwa N, Hirokawa M, Ohyama H, Hata M, Yamada N, et al. Epithelial-myoepithelial carcinoma arising in the nasal cavity. Auris Nasus Larynx. 2008; 35:408–413.
6. Kim HS, Lee WM, Choi SM. Myoepitheliomas of the soft palate: helical CT findings in two patients. Korean J Radiol. 2007; 8:552–555.
7. Harada H, Kashiwagi SI, Fujiura H, Kusukawa J, Morimatsu M. Epithelial-myoepithelial carcinoma--report of a case arising in the nasal cavity. J Laryngol Otol. 1996; 110:397–400.
8. Jin XL, Ding CN, Chu Q. Epithelial-myoepithelial carcinoma arising in the nasal cavity: a case report and review of literature. Pathology. 1999; 31:148–151.
9. Park JO, Jung CK, Sun DI, Kim MS. An unusual presentation of aggressive epithelial-myoepithelial carcinoma of the nasal cavity with high-grade histology. J Laryngol Otol. 2011; 125:1286–1289.
10. Cho KS, Shin SC, Mun MJ, Roh HJ. A case of myoepithelial carcinoma originated from inferior turbinate. Korean J Otorhinolaryngol-Head Neck Surg. 2010; 53:791–794.
11. Lee HM, Choi CS, Kim A, Lee SH. Epithelial-myoepithelial carcinoma arising in the nasal cavity-immunohistochemical and electron microscopic study. Korean J Otolaryngol-Head Neck Surg. 2000; 43:383–386.
12. Cho SH, Kim HT, Kim MS, Sun DI, Koo Y. A clinical study of malignant neoplasms of the nasal septum. Korean J Otolaryngol-Head Neck Surg. 1998; 41:68–72.
13. Compagno J, Wong RT. Intranasal mixed tumors (pleomorphic adenomas): a clinicopathologic study of 40 cases. Am J Clin Pathol. 1977; 68:213–218.
14. Spreer J, Krahe T, Jung G, Lackner K. Spiral versus conventional CT in routine examinations of the neck. J Comput Assist Tomogr. 1995; 19:905–910.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download