Abstract
Middle ear adenoma is a rare benign epithelial tumor. We report the CT and magnetic resonance imaging findings of a case of middle ear adenoma of neuroendocrine differentiation in a 36-year-old man. On high-resolution CT, the mass was found to fill the middle ear, in which the ossicles were embedded, but not destroyed, with outward bulging of the intact tympanic membrane. On MRI, the mass, which was intensely enhanced on 3-dimensional (3D) gadolinium (Gd)-enhanced spoiled gradient-recalled (SPGR) sequence, involved the middle ear, aditus ad antrum and a portion of mastoid antrum. Histological and immunohistochemical findings of the specimen obtained by surgical excisions were consistent with middle ear adenoma of neuroendocrine differentiation. Middle ear adenoma of neuroendocrine differentiation should be included in the differential diagnosis of an intensely enhancing mass filling the middle ear/mastoid antrum without ossicular destructions. The extent of the mass can be excellently assessed with 3D Gd-enhanced SPGR sequence.
Middle ear adenoma is a rare benign epithelial tumor derived from middle ear mucosal cells and can present both a neuroendocrine and an epithelial differentiation (1). Preoperative diagnosis is not possible in most cases, because the clinical features and otoscopic findings are not typical. Although a few cases focusing the imaging features of middle ear adenoma have been reported in the English literature (2, 3), and a few case reports focusing the clinical aspects of middle ear adenoma have appeared in the Korean otolaryngology literature (4-6), imaging features of this rare entity at high-resolution CT and gadolinium (Gd)-enhanced MRI altogether in a single case have rarely been presented in the literature (2). We report herein a case of middle ear adenoma of neuroendocrine differentiation in a 36-year-old man along with CT, MRI and pathologic features.
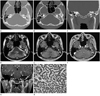
A 36-year-old man presented with aggravated hearing loss of the left ear for the last 5 months. He had experienced mild hearing losses of the left ear without specific management since 10 years prior. Approximately 5 months prior, he had visited local medical clinic with aggravated hearing losses and intermittent otalgia of the left ear, and had medicated under the impression of otomastoiditis without significant symptomatic improvements. He denied otorrhea, dizziness or vertigo and his facial expression was intact. Otoscopic examination revealed a protruding mass behind the intact tympanic membrane (TM). High resolution CT, performed using Sensation 64 scanner (Siemens Healthcare, Forchheim, Germany) with a slice thickness of 0.6 mm, showed a mass filling the middle ear, in which the ossicles were embedded, but not destroyed (Fig. 1A-C). Also being noted were outward bulging of the intact TM (Fig. 1B, C) and complete opacification of the mastoid antrum and air cells (Fig. 1A-C). On the basis of CT findings, we suggested neoplastic conditions or cholesteatoma with associated otomastoiditis. MRI was performed for better evaluations and for better assessments for the extent of the lesion by using a 3.0-T unit (Signa Excite; GE Medical System, Milwaukee, WI, USA). The mass was isointense to gray matter on axial fast spin echo T2-weighted [repetition time (TR)/echo time (TE) = 4800/111; echo train length = 19; matrix number/number of excitation = 512 × 224/3.0; slice thickness = 3.0 mm] (Fig. 1D) and 3-dimensional (3D) spoiled gradient-recalled (SPGR) sequences (TR/TE = 7.9/3.1; flip angle = 20; matrix number/number of excitation = 256 × 224/2; slice thickness = 1.6 mm) (Fig. 1E). In contrast, the opacified mastoid antrum and air cells were hyperintense on both sequences (Fig. 1D, E). Homogeneous and intense enhancement of the mass, involving the entire middle ear cavity, aditus ad antrum, and a portion of mastoid antrum, was noted on Gd-enhanced 3D SPGR sequence (TR/TE = 7.9/3.1; flip angle = 20; matrix number/number of excitation = 256 × 224/2; slice thickness = 1.6 mm) (Fig. 1F, G). In view of CT and magnetic resonance (MR) imaging features, our tentative diagnoses were glomus tympanicum paraganglioma and other neoplastic conditions. He underwent tympanoplasty, canal wall up mastoidectomy, and ossiculoplasty with complete excision of tumor in the middle ear cavity, aditus ad antrum, and mastoid antrum. Intraoperatively, the malleus, incus and stapes superstructures were embedded in the yellowish mass, but they were not destroyed. The incudostapedial articulation was subluxated. The mass did not invade the walls of tympanic cavity nor did it expose the facial nerves. Histologically, the tumor displayed a trabecular growth pattern (Fig. 1H). The tumor cells were cuboidal, and the nuclei were round and uniform. Immunohistochemically, the tumor cells were positive for synaptophysin and chromogranin (not shown). The histological and immunohistochemical findings were consistent with middle ear adenoma of neuroendocrine differentiation. The postoperative audiometric assessment revealed improved hearing. His postoperative course was unremarkable without evidences of recurrence until 14 months after operation.
Two separate adenomatous tumors have been identified in the temporal bone: middle ear adenoma and aggressive papillary tumor (7). The histogenesis of the middle ear adenoma remains controversial, although the consensus tends toward a pluripotential stem cells of the middle ear mucosa as the origin of the lesion. It has been believed that neuroendocrine tumor and middle ear adenoma were separate entities. Recent studies suggest that middle ear adenoma with either epithelial or neuroendocrine differentiation represents opposite ends of a spectrum of differentiation (8). The middle ear adenoma may express synaptophysin, chromogranin, and various polypeptides which are typical for neuroendocrine differentiations (9). Another adenomatous lesion distinct from middle ear adenoma is an aggressive papillary tumor. It is a histologically benign tumor but it clinically shows aggressive growth patterns (10). It expresses cytokeratins, epithelial membrane antigen, and S100.
The middle ear adenoma, a rare benign tumor, is often mistaken for more commonly chronic otomastoiditis or cholesteatoma clinically, as in our case. The overwhelming majority of middle ear adenoma does not invade the temporal bone. Metastasis does not occur (11). The architectural patterns of middle ear adenoma with neuroendocrine differentiation may be glandular, trabecular, or solid. The cells are cuboidal or columnar, and nuclei are round to oval with a "salt and pepper" chromatin pattern and inconspicuous nucleoli. Clinically, the most common symptoms consist of conductive hearing loss, tinnitus and vertigo. Other symptoms include a sense of fullness in the affected ear, otalgia, and, rarely, the facial nerve palsy. The clinical presentation and otoscopic findings are not typical. Thus, the definitive diagnosis is based on histological and immunohistochemical examinations. The imaging features of middle ear adenoma of neuroendocrine differentiation have not yet been clearly defined, because of its relative rarity. Maintz et al. (2) reported CT and/or MR imaging findings of three adenomatous tumors of the middle ear, one adenoma, one adenocarcinoma, and one aggressive papillary tumor, respectively. In all cases, they found a small intratympanic mass in which the ossicles were embedded on high-resolution CT. On MRI, the tumors were isointense or slightly hyperintense compared with white matters on T1-weighted images, and isointense to the gray matters on T2-weighted images, and all cases revealed contrast enhancement. Notably, irrespective of biological behavior of the tumors, none showed destruction of the ossicles or walls of the tympanic cavity. Zan et al. (3) reported high-resolution CT features of a case of middle ear adenoma. They also noticed a well-defined, lobulated, homogeneous, soft tissue-attenuation mass in which the ossicles were embedded, but not destroyed. Despite of fairly large volume of the middle ear adenoma that occupied entire middle ear cavity and a portion of mastoid antrum, the middle ear ossicles were not destroyed but embedded within the mass in our case. The reason why the ossicles are not destroyed but embedded within the tumor in overwhelming majority of the middle ear adenomas is not fully discussed in the literature (2-7). We retrospectively speculated and hypothesized that the soft fragile texture, un-encapsulated with well-delineated outline, and slow growth rate of the middle ear adenoma might be responsible for these findings (3, 5, 7). These typical imaging features of the middle ear adenoma may be helpful in the differentiation of the middle ear adenoma from cholesteatoma, paragangliomas, and malignant tumors of the middle ear cavity.
The middle ear adenoma may present diagnostic dilemma because chronic otomastoiditis and cholesteatoma, which comprise the majority of middle ear diseases, may masquerade middle ear adenoma on high-resolution CT, the most commonly used imaging modality for middle ear diseases (3, 12). Gd-enhanced MRI may be useful in this context. In our case, the extent of the tumor cannot be assessed with high-resolution CT, because the mass and fluid retention in the mastoid antrum and air cells had similar attenuation value, thus could not be distinguished. 3D Gd-enhanced SPGR sequence enabled clear distinction between the intensely enhanced masses in the middle ear, aditus ad antrum and a portion of mastoid antrum, and non-enhancing fluid retention of the mastoid antrum and air cells.
Glomus tympanicum paragangliomas may also mimic middle ear adenoma (3, 13) in that both tumors can show intense enhancements on Gd-enhanced MRI. Zan et al. (3) reported a case of middle ear adenoma whose initial radiologic diagnosis favored glomus tympanicum paraganglioma versus cholesteatoma. Bierry et al. (13) have noticed that a vascular blush was present on angiography in glomus tympanicum paraganglioma, but was absent in middle ear adenoma. A close relationship between the tumor and the Jacobson's nerve or its branches and pulsatile tinnitus were identified in glomus tympanicum paraganglioma, but were absent in middle ear adenoma. Other differential diagnoses include schwannoma of the facial nerves and meningioma.
Complete surgical removal via the external auditory canal or radical mastoidectomy is the treatment of choice.
In summary, middle ear adenoma of neuroendocrine differentiation, a rare entity, should be included in the differential diagnosis when managing inflammatory diseases that does not respond to conservative treatments, or in cases with unclear expansile processes of the middle ear, clinically, and an intensely enhanced mass filling the middle ear/mastoid antrum without ossicular destructions on high-resolution CT and MRI. The extent of the middle ear adenoma of neuroendocrine differentiation can be excellently assessed with high resolution 3D Gd-enhanced SPGR sequences.
Figures and Tables
Fig. 1
CT, MRI, and pathologic features of middle ear adenoma of neuroendocrine differentiation in a 36-year-old man.
A, B. Axial high-resolution CT images show a soft tissue mass filling the middle ear (arrows), in which the ossicles (arrowheads) are embedded, but not destroyed. Also noted are outward bulging of the intact tympanic membrane (open arrow) and complete opacification of the mastoid antrum and air cells (asterisks).
C. Coronal high-resolution CT reveals the mass occupying entire middle ear (arrows). The incus and stapes (arrowheads) are embedded within the mass, but not destroyed.
D, E. Axial fast spin echo T2-weighted (D) and 3-dimensional (3D) spoiled gradient-recalled (SPGR) (E) sequences reveal the mass (arrows), isointense to the gray matter, filling the entire middle ear cavity, aditus ad antrum, and a portion of the mastoid antrum. In contrast, the fluid retention in the mastoid antrum and air cells (asterisks) is hyperintense on both sequences. Note that the hypointense malleus and incus (arrowhead) are embedded within the mass.
F. Axial gadolinium (Gd)-enhanced 3D SPGR sequence demonstrates homogeneous and intense enhancement of the mass (arrows) involving the entire middle ear cavity, aditus ad antrum, and a portion of the mastoid antrum. Fluid retention in the mastoid antrum and air cells (asterisks) shows varying signal intensities and the ossicles (arrowhead) are hypointense.
G. Coronal Gd-enhanced 3D SPGR sequence shows intact incus (arrowhead) embedded within the enhancing middle ear mass (arrows).
H. Histologically, the tumor demonstrates a trabecular growth pattern. The tumor cells are cuboidal, and nuclei are round and uniform (hematoxylin-eosin, × 400).
References
1. Torske KR, Thompson LD. Adenoma versus carcinoid tumor of the middle ear: a study of 48 cases and review of the literature. Mod Pathol. 2002; 15:543–555.
2. Maintz D, Stupp C, Krueger K, Wustrow J, Lackner K. MRI and CT of adenomatous tumours of the middle ear. Neuroradiology. 2001; 43:58–61.
3. Zan E, Limb CJ, Koehler JF, Yousem DM. Middle ear adenoma: a challenging diagnosis. AJNR Am J Neuroradiol. 2009; 30:1602–1603.
4. Shim MJ, Song CI, Yoon TH. A case of middle ear adenoma. Korean J Audiol. 2012; 16:27–30.
5. Shin BS, Kim JR, Jeong HW, Kim EK. Two cases of middle ear adenoma with neuroendocrine differentiation (carcinoid tumor). Korean J Otorhinolaryngol-Head Neck Surg. 2011; 54:573–577.
6. Jun BH, Han YH, Yoon SP, Park SY. A case of the middle ear adenoma. Korean J Otolaryngol-Head Neck Surg. 2000; 43:95–98.
7. Duderstadt M, Förster C, Welkoborsky HJ, Ostertag H. Adenomatous tumors of the middle ear and temporal bone: clinical, morphological and tumor biological characteristics of challenging neoplastic lesions. Eur Arch Otorhinolaryngol. 2012; 269:823–831.
8. Devaney KO, Ferlito A, Rinaldo A. Epithelial tumors of the middle ear--are middle ear carcinoids really distinct from middle ear adenomas? Acta Otolaryngol. 2003; 123:678–682.
9. Wassef M, Kanavaros P, Polivka M, Nemeth J, Monteil JP, Frachet B, et al. Middle ear adenoma. A tumor displaying mucinous and neuroendocrine differentiation. Am J Surg Pathol. 1989; 13:838–847.
10. Gaffey MJ, Mills SE, Fechner RE, Intemann SR, Wick MR. Aggressive papillary middle-ear tumor. A clinicopathologic entity distinct from middle-ear adenoma. Am J Surg Pathol. 1988; 12:790–797.
11. Saliba I, Evrard AS. Middle ear glandular neoplasms: adenoma, carcinoma or adenoma with neuroendocrine differentiation: a case series. Cases J. 2009; 2:6508.
12. Tomazic PV, Beham A, Lackner A, Ropposch T, Stockreiter U, Walch C. Neuroendocrine adenoma of the middle ear (NAME) mimicking as chronic otitis media with an episode of facial nerve palsy. B-ENT. 2011; 7:121–125.
13. Bierry G, Riehm S, Marcellin L, Stierlé JL, Veillon F. Middle ear adenomatous tumor: a not so rare glomus tympanicum-mimicking lesion. J Neuroradiol. 2010; 37:116–121.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download