Abstract
Intrahepatic cholangiocarcinoma (ICC) is a rare malignancy and many cases of ICCs are diagnosed in an unresectable state. Until now there has been no effective palliative treatment for these cases. Recently yttrium-90 (90Y) radioembolization has been highlighted as a new palliative treatment for these cases. In Korea, there has been no reported case of unresectable ICC which was treated by 90Y radioembolization up until now. We treated two cases of unresectable ICCs with 90Y radioembolization and the ICCs were necrotized effectively without significant toxicity.
Intrahepatic cholangiocarcinoma (ICC) is an epithelial cancer which arises from the intrahepatic bile duct. Surgical resection or liver transplantation is the only known curative treatment for ICC (1). However, most of the patients visit hospital too late due to asymptomatic progression of the tumor to be treated curatively. Because there is no established effective palliative treatment option for unresectable ICC, median survival duration of unresectable ICC is only 6-12 months (2).
For this reason, variable palliative treatments have been tried on patients with unresectable ICCs. Systemic chemotherapy with the combination of gemcitabine and cisplatin showed poor results in treating patients with unresectable ICC. The overall survival was still less than 1 year (2). Several trials on the effects of transarterial chemoembolization (TACE) also showed a wide range of the relatively short median survival from 12.2-15 months (3-5). Recently, chemoembolization with drug-eluting beads-TACE was investigated, but the median overall survival was also short - 11.7 months (6). Despite the development of stereotactic body radiotherapy, median survival of patients receiving external beam radiation therapy for unresectable ICC was 10.6 months and patients experienced some complications such as biliary stenosis or liver failure (7).
Herein, yttrium-90 (90Y) radioembolization is highlighted as an effective palliative treatment in patients with unresectable ICC without significant adverse effects. 90Y-bearing microspheres are the point radiation-source which release pure high-energy β-ray localized in the intratumoral arterial vasculature. Mean penetrating length is 2.5 mm and maximum penetrating length is 11 mm in soft tissue (8). 90Y-bearing microspheres can necrotize the tumor only, without extensive hepatic parenchymal damage when implanted in the tumor supplying artery.
We performed transarterial 90Y radioembolization treatment on two patients with unresectable ICCs and the malignant lesions were necrotized effectively by this new treatment without significant toxicity. This is the first report of transarterial 90Y radioembolization treatment of ICCs as a palliative option in Korea.
A 38-year-old man presented with a 2-week history of severe fatigue. His medical history was not unusual except for a 5-year history of diabetes mellitus. Liver function tests were in the normal range. An abdominal CT scan showed an 18 cm mass occupying most of the right hepatic lobe and segment 4 (Fig. 1A). We did sonography-guided biopsy for this lesion and it revealed ICC. At the time of the diagnosis, ICC was in an unresectable state, stage 4, due to its huge size although there was no evidence of distant metastasis. There were no significant abnormalities in hepatic and renal functions and total bilirubin was measured at 1.36 mg/dL. We planned transarterial 90Y radioembolization treatment as a palliative treatment. Before 90Y bead implantation, we did a Technetium 99m macroaggregated albumin (99mTc-MAA) scan for evaluation of the hepatopulmonary (HP) shunt and tumor-to-normal (T/N) ratio (Fig. 1B). Results of the examination showed that the HP shunt rate was 5.87% (Table 1). We determined that the maximum radiation dose for the tumor within the limitation of that HP shunt did not exceed 20% and the normal liver dose did not exceed 100 Gy. First, we did hepatic arteriography (Fig. 1C). After that, we implanted 2.94 GBq and 0.84 GBq of resin 90Y microsphere (Sirspheres@, Sirtex, Sydney, Australia) into the right and left hepatic arteries, respectively.
After 18-months of 90Y radioembolization, the follow-up contrast enhanced CT scan at the arterial phase showed that the size of the lesion was reduced (18 cm to 15 cm) and the internal necrotic portion of the tumor had increased (Fig. 1D). Also the follow-up positron emission tomography (PET)-CT scan showed total disappearance of the previously noted hot-uptake lesion (Fig. 1E), and there was no new hot-uptake lesion in the liver (Fig. 1F). So we believed that the tumor was necrotized effectively. The patient did not complain of any symptoms and did not show significant toxicities above grade 3 (Table 2).
A 74-year-old man presented with weight loss of 5 kg in one year. His medical history was not unusual except for well controlled hypertension. Liver function tests were in the normal range. Contrast enhanced abdominal CT scan showed a 5.5 cm sized homogeneously well enhanced lesion in S1, S7 and S8 (Fig. 2A). We did sonography-guided biopsy for this lesion and it revealed ICC. At the time of the diagnosis, ICC was in an unresectable state. There were no significant abnormalities in hepatic and renal functions and total bilirubin was measured at 0.3 mg/dL. We planned transarterial 90Y radioembolization treatment as a palliative treatment. Before the 90Y bead implantation, we did a 99mTc-MAA scan (Fig. 2B). Results of the examination showed that the HP shunt rate was 4.12%, so we could do the treatment without a radiation dose reduction (Table 1). After we superselected the supplying artery of the tumor in S1 and the right hepatic artery (Fig. 2C), we implanted 0.5 GBq and 0.7 GBq of the resin 90Y microspheres (Sirspheres@, Sirtex, Sydney, Australia), respectively.
After 25-month 90Y radioembolization, a follow-up contrast enhanced CT scan at the arterial phase showed that the size of the lesion was reduced (5.5 cm to 3 cm) and most of the tumor had necrotized (Fig. 2D). Also, the follow-up PET-CT showed total disappearance of the previously noted hot-uptake lesion (Fig. 2E, F). So we thought that the tumor had necrotized effectively. The patient did not complain of any symptoms and did not show significant toxicities over grade 3 (Table 2).
In the USA, the prevalence of cholangiocarcinoma is 0.32 cases per 100000 people in 1975-1979, and it has increased rapidly to 0.85 cases per 100000 people in 1995-1999 (9). In Korea, in 1999, already the prevalence of the cholangiocarcinoma was already 3.6 cases per 100000 people because of the high infection rate with Clonorchis sinensis due to the high rate of ingestion of freshwater fish in Korea (10). In addition, the prevalence of cholangiocarcinoma in Korea has shown rapid growth trends, so in 2005, 5.2 people per 100000 were reported as having cholangiocarcinoma (10).
Recently 90Y radioembolization has been highlighted as effective palliative treatment in patients with unresectable ICC without significant adverse effects. US FDA permitted 90Y radioembolization treatment in unresectable primary hepatic cellular carcinoma and metastatic hepatic carcinoma from colon cancer, and several clinical studies have shown good results. Three small group studies were conducted on the effects of 90Y radioembolization treatment on unresectable ICCs in 2008, 2010 and 2011. Those studies showed mean survival duration of 14.9, 9.3 and 10 months, respectively, without significant toxicities (11-13).
Before 90Y radioembolization, a 99mTc-MAA scan should be done for radiation dose selection. The 99mTc-MAA scan shows the rate of flow in the HP shunt and the T/N uptake ratio of the 90Y microspheres. First, the rate of the shunt between hepatic artery and pulmonary vessel should be under the 20% because of radiation pneumonitis caused by refluxed 90Y microspheres. Second, the selective delivery of radiation to the tumor with sparing of non-tumorous liver parenchyma depends on the T/N uptake ratio of the 90Y microspheres (14). In general, we determine the maximum radiation dose for the tumor within the limitation of that HP shunt is not exceed 20% and the normal liver dose should not exceed 100 Gy.
Until now, several adverse effects after treatment have been reported. Most of these are caused by unwanted implantation of 90Y microspheres by reflux or the shunt. Gastroduodenal ulceration can develop when there is reflux of 90Y microspheres into the gastroduodenal artery, and radiation pneumonitis can develop if the microspheres become implanted in the lung through the HP shunt. To avoid these toxic effects, coiling of the gastroduodenal artery and exact evaluation of the HP shunt should be done.
In general, the Response Evaluation Criteria in Solid Tumors criteria (15) is used in systemic chemotherapy or general radiation therapy. However it has limitations in evaluating the effects of 90Y radioembolization. Because the main mechanism of treatment is micro-infarction and embolization, 90Y radioembolization induces necrosis with or without change in tumor size. For this reason, PET-CT is a more useful modality to evaluate the treatment response after 90Y radioembolization because it shows metabolic activities. A tumor that has been treated effectively does not show hot-uptake, although it does not show decrease in size (8). In our case (case 1), there was no considerable decrease in size, but the follow-up PET-CT showed total disappearance of the hot-uptake portion.
90Y radioembolization treatment for unresectable cholangiocarcinoma is still in the experimental stage, but several studies including our study showed promising results. If this palliative treatment is performed on appropriate patients with thorough pre-assessment, we can obtain good results without specific toxicity. In the future, if the problem of the high cost can be resolved, 90Y radioembolization treatment can be the most effective palliative treatment modality in unresectable intrahepatic cholangiocarcinoma.
Figures and Tables
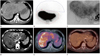 | Fig. 1A 38-year-old man with unresectable intrahepatic cholangiocarcinoma.
A. Initial abdominal CT scan (arterial phase) shows 18 cm sized huge mass lesion with peripheral arterial enhancement in both hepatic lobes.
B. Technetium 99m macroaggregated albumin scan shows low hepatopulmonary shunt rate (5.87%) and high tumor-to-normal ratio (7.89).
C. Celiac arteriography shows hypervascular tumor in both hepatic lobes.
D-F. At the 18-month after treatment, size of lesion is reduced (15 cm) and internal necrotic portion is increased. At the 18-month after treatment, previously noted hot-uptake lesion (E) is no more detected in positron emission tomography-CT scan (F).
|
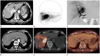 | Fig. 2A 74-year-old man with unresectable cholangiocarcinoma.
A. Contrast enhanced CT scan at arterial phase shows 5.5 cm sized intrahepatic cholangiocarcinoma in S1, S7 and S8 before treatment.
B. Technetium 99m macroaggregated albumin scan shows low hepatopulmonary shunt rate (4.12%) and high tumor-to-normal ratio (8.08).
C. Celiac arteriography shows hypervascular tumor.
D-F. At the 25-month after treatment, size of lesion is reduced (3.0 cm) and internal necrosis is noted. At the 25-month after treatment, previously noted hot-uptake lesion (E) is no more detected in positron emission tomography-CT scan (F).
|
References
1. Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008; 48:308–321.
2. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–1281.
3. Kiefer MV, Albert M, McNally M, Robertson M, Sun W, Fraker D, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer. 2011; 117:1498–1505.
4. Vogl TJ, Naguib NN, Nour-Eldin NE, Bechstein WO, Zeuzem S, Trojan J, et al. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: results and prognostic factors governing treatment success. Int J Cancer. 2012; 131:733–740.
5. Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011; 66:322–328.
6. Kuhlmann JB, Euringer W, Spangenberg HC, Breidert M, Blum HE, Harder J, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol. 2012; 24:437–443.
7. Kopek N, Holt MI, Hansen AT, Høyer M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother Oncol. 2010; 94:47–52.
8. Murthy R, Nunez R, Szklaruk J, Erwin W, Madoff DC, Gupta S, et al. Yttrium-90 microsphere therapy for hepatic malignancy: devices, indications, technical considerations, and potential complications. Radiographics. 2005; 25:Suppl 1. S41–S55.
9. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001; 33:1353–1357.
10. Shin HR, Oh JK, Lim MK, Shin A, Kong HJ, Jung KW, et al. Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in Korea. J Korean Med Sci. 2010; 25:1011–1016.
11. Ibrahim SM, Mulcahy MF, Lewandowski RJ, Sato KT, Ryu RK, Masterson EJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer. 2008; 113:2119–2128.
12. Saxena A, Bester L, Chua TC, Chu FC, Morris DL. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010; 17:484–491.
13. Hoffmann RT, Paprottka PM, Schön A, Bamberg F, Haug A, Dürr EM, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012; 35:105–116.
14. Ho S, Lau WY, Leung TW, Chan M, Chan KW, Lee WY, et al. Tumour-to-normal uptake ratio of 90Y microspheres in hepatic cancer assessed with 99Tcm macroaggregated albumin. Br J Radiol. 1997; 70:823–828.
15. van Persijn van Meerten EL, Gelderblom H, Bloem JL. RECIST revised: implications for the radiologist. A review article on the modified RECIST guideline. Eur Radiol. 2010; 20:1456–1467.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download