Abstract
Lipomatous hemangiopericytoma (LHPC) is recently recognized as a rare hemangiopericytoma variant. To our knowledge, imaging features of LHPC involving skull base have not yet been reported. We present the imaging features of LHPC of skull base in a 44-year-old female, along with a literature review CT and magnetic resonance imagings showed well-enhanced fatty issues containing temporal skull base masses, with pressure bony erosions.
Hemangiopericytomas were described as tumors arising from the pericytes of Zimmermans, which are modified smooth muscle contractile cells surrounding the capillaries (1). Hemangiopericytoma is a rare tumor with an incidence of less than 1% of all central nervous system (CNS) tumors. In addition, hemangiopericytoma is an aggressive tumor with malignant potential and tends to recur and occasionally metastasize, mainly to the bone, lung, and liver (2).
However, hemangiopericytomas with a significant lipomatous component were first introduced into literature in 1990. Later, Nielsen et al. (3) coined the term, lipomatous hemangiopericytoma (LHPC), as a pathologically distinct entity. Folpe et al. (4) reported LHPC that occurred in adults, primarily in the deep soft tissues of the leg and in the retroperitoneum.
This entity, arising from the skull base has not yet been documented on magnetic resonance (MR) imaging. We experienced a surgically proven LHPC of the temporal skull base within a 44-year-old woman.
A 44-year-old woman with no significant medical history was presented with a 1-month history of dizziness.
On CT scan, the well-enhanced hypervascular extra-axial tumor containing intratumoral irregular fatty attenuation, the lipoma component, was shown at the right temporal base area (Fig. 1A). In addition, directly extracranial extension via widened foramen ovale due to bony eruption was observed (Fig. 1B, C).
On MRI, the mass was hypointense on T1-weighted images, and high signal intensity on T2-weighted images with intratumoral signal void vascular structures. The mass showed irregular central high signal intensity portions on both T1-weighted and T2-weighted images, intratumoral lipoma component (Fig. 1D-F), and some inferior extension to the extracranial space via the foramen ovale, with mild thinning of the lateral wall of the right sphenoid sinus and direct extrinsic compression to the adjacent right cavernous sinus, and right Meckel's cave (Fig. 1G). Angiography revealed a hypervascular mass, fed by the right middle meningeal artery and internal maxillary artery (Fig. 1H).
On histopathology, this tumor showed characteristics of high vascularity, solid nests of cells with hyperchoromatic nuclei, and adipocytes within the tumor matrix (Fig. 1I). She was discharged in stable condition after 19 days of her hospital stay.
Intracranial hemangiopericytomas are neoplasm of pericytes that originate in the meninges, without meningioma component. Hemangiopericytomas represent less than 1% of all CNS tumors (5). It arise from perivascular pericytes and can be graded as differentiated [World Health Organization (WHO) grade II] and anaplastic (WHO grade III) tumors.
These are aggressive lesions that tend to occur at an earlier age than the other meningeal tumors, recurring with high frequency, and metastasize extracranially.
Servo et al. (6) described their CT findings of intracranial hemangiopericytomas as follows: unenhanced CT scans showed isodense or slightly hyperdense, well-defined nodular mass, without calcification, and these lesions were associated with slight edema. In addition, hemangiopericytomas are associated with narrow-based dural attachment and bone erosion, but hyperostosis is not presented (7).
Chiechi et al. (1) described their MR findings of intracranial hemangiopericytomas as follows: intracranial hemangiopericytomas were heterogeneous, predominantly isointense on T1-weighted and T2-weighted images, with intratumoral signal void vascular structure and were well-enhanced heterogeneously on contrast-enhanced MR images.
The recently described variant, LHPC, is highly rare. To date, 49 cases of LHPC have been documented in published reports in the English language (8).
This tumor is similar to hemangiopericytoma, having the same age and anatomic distribution; however, pathologically, lipomatous hemangiopericytoma demonstrates variable amounts of distinct mature adipose cells (5).
Their biological behavior has yet to be determined. In 1995, by Nielsen et al. (3), LHPC appears histologically benign and reports suggest benign clinical behaviors. Almost all tumors have low or absent mitotic activity: only 6% (2/33) of the tumor reported had more than 4 mitoses per high power field.
In 1999, Folpe et al. (4) reported lipomatous hemangiopericytoma occurring in adults, primarily in the deep soft tissues of the leg and in the retroperitoneum. However, tumor developing in the skull base area is very unusual.
Meningioma should be distinguished. In a review of 21 intracranial hemangiopericytomas by Jääskeläinen et al. (9), only eight tumors imaged by CT showed broad-based dural attachments. However, Buetow et al. (10) reported that up to 85% of meningiomas in their series showed a broad base of dural attachment. In our case, LHPC showed a relatively narrow base of dural attachment, a feature not typically found in meningiomas.
Meningiomas are also commonly described as dural-based tumors that are frequently associated with hyperostosis and calcification. Unlike meningioma, our case showed adjacent bone erosion and did not have tumor calcification.
Wide local extirpation is recommended, with adjuvant radiation and chemotherapy reserved for malignant variation or metastatic disease.
To our knowledge, this is the first reported case traversing the skull base. More cases of LHPC of the skull must be studied to draw definitive conclusions about its distinct behavior and management.
Figures and Tables
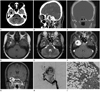 | Fig. 1A 44-year-old woman who presented with 1 month history of dizziness.
A. Precontrast CT image shows well-defined round solid increased attenuated tumor (arrowheads), with containing intratumoral fat-attenuated (-60 Hounsfield unit) component (arrow) at the right temporal base area.
B. Sagittal postcontrast CT image shows well enhancing solid hypervascular extraaxial tumor with small inferior extracranial extention via widened foramen ovale.
C. Coronal temporal bone CT image shows tumor with adjacent bone pressure erosion and small inferior extracranial extention via widened foramen ovale (arrows).
D. T1-weighted axial magnetic resonance (MR) image shows slightly hypointense mass (arrows) with central irregular high signal intensity portions, intratumoral lipoma component (arrowheads).
E. T2-weighted axial MR image shows intermediate signal intensity mass, intratumoral signal void structures (arrows), with intratumoral high signal intensity portions (arrowheads).
F. Contrast-enhanced T1-weighted axial MR image shows well enhancing solid mass.
G. Contrast-enhanced T1-weighted coronal MR image shows small inferior extension to extracranial space via foramen ovale with central fat-saturated poorly enhancing portion (arrowheads).
H. Lateral angiography shows hypervascular tumor staining fed by the right middle meningeal artery (arrowheads), and internal maxillary artery (arrows).
I. Histologic features of lipomatous hemangiopericytoma. Microscopic view shows a highly vascular tumor, with hyperchromatic nuclei and normal adipocytes are seen within the tumor matrix (H&E, × 200).
|
References
1. Chiechi MV, Smirniotopoulos JG, Mena H. Intracranial hemangiopericytomas: MR and CT features. AJNR Am J Neuroradiol. 1996; 17:1365–1371.
2. Fountas KN, Kapsalaki E, Kassam M, Feltes CH, Dimopoulos VG, Robinson JS, et al. Management of intracranial meningeal hemangiopericytomas: outcome and experience. Neurosurg Rev. 2006; 29:145–153.
3. Nielsen GP, Dickersin GR, Provenzal JM, Rosenberg AE. Lipomatous hemangiopericytoma. A histologic, ultrastructural and immunohistochemical study of a unique variant of hemangiopericytoma. Am J Surg Pathol. 1995; 19:748–756.
4. Folpe AL, Devaney K, Weiss SW. Lipomatous hemangiopericytoma: a rare variant of hemangiopericytoma that may be confused with liposarcoma. Am J Surg Pathol. 1999; 23:1201–1207.
5. Shaia WT, Bojrab DI, Babu S, Pieper DR. Lipomatous hemangiopericytoma of the skull base and parapharyngeal space. Otol Neurotol. 2006; 27:560–563.
6. Servo A, Jääskeläinen J, Wahlström T, Haltia M. Diagnosis of intracranial haemangiopericytomas with angiography and CT scanning. Neuroradiology. 1985; 27:38–43.
7. Chen Q, Chen XZ, Wang JM, Li SW, Jiang T, Dai JP. Intracranial meningeal hemangiopericytomas in children and adolescents: CT and MR imaging findings. AJNR Am J Neuroradiol. 2012; 33:195–199.
8. Jing HB, Meng QD, Tai YH. Lipomatous hemangiopericytoma of the stomach: a case report and a review of literature. World J Gastroenterol. 2011; 17:4835–4838.
9. Jääskeläinen J, Servo A, Haltia M, Wahlström T, Valtonen S. Intracranial hemangiopericytoma: radiology, surgery, radiotherapy, and outcome in 21 patients. Surg Neurol. 1985; 23:227–236.
10. Buetow MP, Buetow PC, Smirniotopoulos JG. Typical, atypical, and misleading features in meningioma. Radiographics. 1991; 11:1087–1106.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download