Abstract
Purpose
To analyze the bone mineral density (BMD) in multiple myeloma (MM) and to compare BMD with plain radiography, MRI and clinical stage.
Materials and Methods
We reviewed 59 patients with MM and an age- and sex-matched control group, with measured BMD. The L-spine and femoral neck (FN) BMD were measured by dual-energy X-ray absorptiometry. Lateral plain radiographs of the L-spine were graded as 3 stages using the modified Saville index. Four bone marrow patterns were classified on sagittal T1- and T2-weighted magnetic resonance images of the L-spine. BMD in the MM and control group were analyzed. BMD in MM was compared with the modified Saville index, bone marrow patterns on MRI, and clinical stages.
Results
In MM, spine BMD was reduced and the difference between spine and FN BMD was larger than the control group (p < 0.001). The modified Saville index was negatively correlated with spine T scores (p < 0.01). The spine BMD in normal marrow pattern on the MRI was the most reduced. There was no statistical correlation between BMD and clinical stage.
Multiple myeloma (MM) is characterized by a widespread proliferation of monoclonal plasma cells in the bone marrow. The common clinical features of MM are bone destruction, including osteolytic lesions, fracture, vertebral collapse, and osteoporosis.
Bone mineral density (BMD) measured by dual-energy X-ray absorptiometry (DXA) is used to diagnose osteoporosis and assess fracture risk. DXA is a diagnostic technique based on low dose radiation that is non-invasive, has short scan times, involves a quick setup for patients, and has good measurement precision (1). It provides a value of BMD which can be compared with an age- and sex-matched reference range.
The Saville index has been a widely used method for the grading of osteoporosis in daily practice (2). While osteoporosis is one of the major features of MM, there has been no study that the Saville index can be used for assessing osteoporosis even in MM to our knowledge.
We wondered whether the pattern or extent of myeloma cell involvement on magnetic resonance (MR) imaging was correlated with the degree of osteoporosis. There has been only one study comparing the MR pattern with the degree of osteoporosis in MM (3).
The purpose of our study was to analyze the BMD in patients with MM and compare the BMD value with a grade of osteoporosis on plain radiograph, the bone marrow pattern on the MR image, and the clinical stage.
We retrospectively reviewed 113 patients with newly diagnosed MM between 2005 and 2012 in our institute. Among them, 67 patients who had BMD measurements and who underwent plain radiography and MR imaging of the lumbar spine were selected. Six patients with a metal prosthesis or bone cement insertion in the spine were excluded from the analysis. Two patients were excluded due to an inadequate laboratory test. An age and sex matched control group with BMD measurements obtained for health-screening purposes was also collected.
In both the study and control groups, BMD (g/cm2) was measured in the spine (L1-L4, antero-posterior view) and femoral neck by a DXA (Hologic, Inc., Bedford, MA, USA). Lateral plain radiographs of the lumbar spine were classified as three grades using a modified Saville index (grade 1, minimal loss of density; grade 2, more obvious vertical striation and concave endplate; grade 3, more severe loss of bone density than grade 2, greater reduction in height, or collapsed vertebral body).
MR imaging was taken by a 3.0-T and 1.5-T MR systems (Achieva, Philips Medical Systems, Best, The Netherlands; Signa HDxt, GE Healthcare, Milwaukee, WI, USA; Signa Excite, GE Healthcare, Milwaukee, WI, USA). All patients underwent sagittal fat-suppressed T2-weighted MR images, sagittal T1-weighted MR images, and axial T1- and T2-weighted images of the lumbar spine. Among them, sagittal T1- and fat-suppressed T2-weighted images were evaluated in this session.
Two radiologists specializing in the musculoskeletal system assessed the bone marrow infiltrative pattern in consensus. Four different bone marrow patterns on MR imaging could be differentiated. Bone marrow with a hyperintense signal compared with the disc on T1-weighted images and a hypointense signal compared to the muscle tissue on T2-weighted images was regarded as normal (type I) (4). The bone marrow pattern was described as a multiple focal pattern if any T1-weighted hypointense and T2-weighted hyperintense signal lesions were detected (type II). Bone marrow demonstrating a very inhomogeneous patchy pattern on both T1- and T2-weighted images was described as a so-called "salt-and-pepper" pattern (type III). Fourth, a diffuse infiltration pattern was defined by the presence of a homogenous hypointense change in the whole spine, similar or lower than the disc signal intensity on T1-weighted images with a hyperintense signal on T2-weighted images (type IV). The clinical stage was graded by the Durie-Salmon staging system (DSS) (5) and the international staging system (ISS) (6).
A t-test was used to compare the difference between the spine and femoral neck BMD in both the study and control groups. The BMD values in MM were compared with the grade by the modified Saville index, the bone marrow patterns on MR imaging, and the clinical stages, using one-way and repeated measures analysis of variance. Statistical analysis was performed using the SPSS 10.0/PC computer program (SPSS Inc., Chicago, IL, USA).
Out of 113 patients, 59 were enrolled in this study. They consisted of 28 men and 31 women (mean age, 66 years; range, 40-86 years). An age- and sex-matched control group of 59 subjects was also collected.
In patients with MM, the spine BMD T score was -2.7 ± 1.5 (mean ± SD, g/cm2) and Z score was -1.3 ± 1.3 and femoral neck BMD T score was -1.6 ± 1.2 and Z score was -0.6 ± 1.0. In the control group, spine BMD T score was -1.6 ± 1.2 and Z score was -0.3 ± 1.2 and femoral neck BMD T score was -1.5 ± 0.9 and Z score was -0.5 ± 0.9 (Table 1).
The spine BMD was significantly reduced in MM patients compared to the control group (p < 0.001). The difference between spine and femoral neck BMD (T score 1.0 ± 1.0, Z score 0.7 ± 0.9) in MM was significantly greater than that in the control group (T score 0.2 ± 0.9, Z score 0.2 ± 0.9) (p < 0.001) (Fig. 1).
According to the modified Saville index, plain radiographs of the patients with MM were classified as grade 1 (n = 24), grade 2 (n = 20), and grade 3 (n = 15) (Fig. 2). The results of the modified Saville index were negatively correlated with the spine BMD T scores (r = 0.3, p < 0.01).
With regard to the analysis of the bone marrow pattern on MR imaging, 12 patients showed type I (Fig. 3), 14 type II (Fig. 4), 20 type III (Fig. 5) and 13 type IV (Fig. 6). Compared to MR images, the BMD in the type I and type III was lower than the BMD in the other 2 patterns (p < 0.5), with the BMD in the type I on MR images showing the greatest decrease (Table 2).
There was no statistical correlation between BMD and clinical stages by DSS and ISS.
The clinical features of MM result from the proliferation of B cells characterized by bone marrow infiltration and the production of monoclonal immunoglobulin. While myeloma cells induce increased osteoclast activation and osteoclastic bone resorption, the effects on the osteoblasts are more complex, leading to painful osteolytic destruction and pathologic fractures (7, 8).
Demineralization and lytic bone lesions in MM can be assessed by plain radiography, computed tomography, MR imaging, and DXA. Among them, DXA is a sensitive, noninvasive, and relatively inexpensive technique for the diagnosis and follow-up of metabolic bone disease such as osteoporosis (9-11).
Since MM is one of the etiologies of secondary osteoporosis, the efficacy of the measurement of BMD by DXA in MM has previously been reported. Some studies have suggested that an increased risk of early vertebral collapse could be predicted by DXA (7, 12). Other studies have reported that measuring the BMD value by DXA can be used as a marker of treatment response in MM (11, 13). Another study supported an advantage of DXA in predicting the prognosis in the asymptomatic stage I MM (3). In addition, low lumbar BMD correlated with the severity of the radiological finding at the time of diagnosis (14).
In our study, measuring BMD by DXA was useful in distinguishing certain characteristics of MM. Compared to the control group spine BMD in MM was significantly diminished. Femoral neck BMD was not significantly different in both groups. Hence, in MM, the difference between spine BMD and femoral neck BMD was larger than that in the control group. This discrepancy of spine and femoral neck BMD in MM has been rarely reported previously. One study reported that lumbar BMD was often diminished in MM at diagnosis, whereas femoral neck BMD was rarely affected (14).
Discordance between spine and femoral neck BMD could also be present in the normal population (1). A possible explanation is the varying proportion of cancellous and cortical bone at the different sites. First, cancellous bone, which represents 20% of the total bone mass, has an accelerated metabolism and therefore a more rapid and earlier loss than cortical bone. This could be an important explanation for the presence of a lower spine BMD in the relatively young population in the early postmenopausal period (1). Second, most of the causes for secondary osteoporosis first affect the spine, leading to a higher prevalence of lumbar spine osteoporosis. Third, there is the possibility that weight bearing can cause a rise in BMD, especially in the femoral neck.
In our study, femoral neck BMD was slightly higher than spine BMD in the control group; however, the difference between spine and femoral neck BMD in the control group was quite smaller than in patients with MM. This suggests that MM may involve the spine much more such that, with its high proportion of cancellous bone, it may be more prone to osteoporosis.
Generally, the Saville index has been used for the scoring of spinal osteopenia from grade 0 to grade 4 (2). In this study, the spine was graded by a modified Saville index to improve inter- and intra-observer agreement between the original Saville index grade 1 and grade 2. In our study, the grades by the modified Saville index in MM were correlated with the T-score by DXA. We therefore concluded that the modified Saville index on plain radiography was a valuable grading system for osteoporosis in MM in daily practice.
In theory, a collapsed vertebral body may increase BMD per unit area and thus result in a falsely elevated BMD value. However, in our study, spine BMD in MM with a modified Saville index grade 3 indicating vertebral collapse tended to reduce compared to grade 1 or 2. Consistent with a previous study (14), we concluded that demineralization and loss of trabecular bone in MM may not lead to bone condensation in a collapsed vertebra. Therefore, we agreed that when using DXA in patients with MM, collapsed vertebrae need not be excluded from the analysis.
Recently, MR imaging has been used for diagnosis, staging, and assessing the treatment response in MM. To our knowledge, there has only been one report comparing BMD and MR imaging patterns in MM. Mariette et al. (3) suggested that, in asymptomatic patients with stage I MM, spine BMD values were similar between the two groups that had initial normal and abnormal MR findings. An abnormal MRI pattern did not correlate with the degree of osteoporosis evaluated by spine BMD in stage I MM.
In our study, the bone marrow pattern on MR imaging was classified as normal, multiple focal, salt-and-pepper, and diffuse infiltrative (8, 15-18). We compared the bone marrow patterns on MR imaging to the BMD. We found that BMD with a normal marrow pattern tended to be lower than other patterns. A normal marrow pattern reveals as minor microscopic plasma cell infiltration (< 20 volume% in bone marrow biopsy) (8). These patients are eligible for a "watch and wait" strategy. Although MR imaging is a sensitive modality for detecting bone marrow lesions, we concluded that the MR imaging pattern did not correlate with the status of mineralization. Further, in MM, the general MR pattern cannot predict fracture risk because of secondary osteoporosis.
In our study, the clinical stage in MM using the DSS and ISS was not correlated with spine and femoral neck BMD. However, because a strong correlation with vertebral fracture and low spine BMD has been suggested in previous studies (7, 14), we agree that BMD measurements are still useful in the initial diagnosis of MM in addition to monitoring after treatment.
There were several limitations in our study. First, the evaluation of MR imaging patterns was difficult owing to the usage of 3 different MR machines. Nonetheless, 2 radiologists specializing in the musculoskeletal system reached a consensus concerning bone marrow patterns. Second, BMD values were not adjusted to reflect age-related degenerative changes and, therefore, relied on comparisons with BMD values in the control group. Third, other disease processes that would lead to a discordance between femoral neck and spin BMD values could not be excluded. Fourth, our small sample size could have affected the observed decrease in BMD in the normal marrow pattern on MR imaging.
In MM, spine BMD was significantly reduced and the difference between spine and femoral neck BMD was larger than in the control group. Multiple myeloma should therefore be included in the differential diagnosis when the discrepancy between spine and femoral neck BMD in patients with osteoporosis is detected. The modified Saville index was significantly correlated with BMD in MM. The modified Saville index may be considered as a useful method for assessing osteoporosis in MM. The BMD with a normal marrow pattern on MR imaging tended to be lower than in other patterns. Furthermore, the BMD did not correlate with the clinical stage in MM.
Figures and Tables
 | Fig. 1BMD in MM and control group.
A. Spine BMD was significantly reduced in MM patients compared to the control group (p < 0.001).
B. The difference between spine and femoral neck BMD in MM was larger than that in the control group (p < 0.001).
Note.-BMD = bone mineral density, MM = multiple myeloma
|
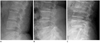 | Fig. 2Modified Saville index.
A. Lateral plain radiograph of L-spine shows minimal decreased bone density indicating modified Saville index grade 1.
B. More obvious vertical striation with concave endplates reveals modified Saville index grade 2.
C. Severe diminished bone density and a more collapsed vertebral body indicates modified Saville index grade 3.
|
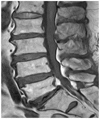 | Fig. 3Normal marrow pattern on magnetic resonance images. Bone marrow shows hyperintense signal compared with the disc on T1-weighted images. |
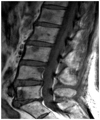 | Fig. 4Multiple focal pattern on magnetic resonance images. Multifocal T1-weighted hypointense lesions are detected in the L-spine. |
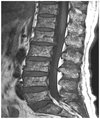 | Fig. 5Salt-and-pepper pattern. The bone marrow shows a very inhomogeneous patchy signal on T1-weighted images. |
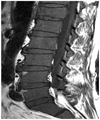 | Fig. 6Diffuse infiltrative pattern. Homogenous hypointense change which is similar or lower than disc signal intensity on T1-weighted images is shown in the spine. |
References
1. Mounach A, Abayi DA, Ghazi M, Ghozlani I, Nouijai A, Achemlal L, et al. Discordance between hip and spine bone mineral density measurement using DXA: prevalence and risk factors. Semin Arthritis Rheum. 2009. 38:467–471.
2. Saville PD. A quantitative approach to simple radiographic diagnosis of osteoporosis: its application to the osteoporosis of rheumatoid arthritis. Arthritis Rheum. 1967. 10:416–422.
3. Mariette X, Zagdanski AM, Guermazi A, Bergot C, Arnould A, Frija J, et al. Prognostic value of vertebral lesions detected by magnetic resonance imaging in patients with stage I multiple myeloma. Br J Haematol. 1999. 104:723–729.
4. Baur A, Stäbler A, Bartl R, Lamerz R, Scheidler J, Reiser M. MRI gadolinium enhancement of bone marrow: age-related changes in normals and in diffuse neoplastic infiltration. Skeletal Radiol. 1997. 26:414–418.
5. Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975. 36:842–854.
6. Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005. 23:3412–3420.
7. Abildgaard N, Brixen K, Eriksen EF, Kristensen JE, Nielsen JL, Heickendorff L. Sequential analysis of biochemical markers of bone resorption and bone densitometry in multiple myeloma. Haematologica. 2004. 89:567–577.
8. Lütje S, de Rooy JW, Croockewit S, Koedam E, Oyen WJ, Raymakers RA. Role of radiography, MRI and FDG-PET/CT in diagnosing, staging and therapeutical evaluation of patients with multiple myeloma. Ann Hematol. 2009. 88:1161–1168.
9. Dhodapkar MV, Weinstein R, Tricot G, Jagannath S, Parfitt AM, Manolagas SC, et al. Biologic and therapeutic determinants of bone mineral density in multiple myeloma. Leuk Lymphoma. 1998. 32:121–127.
10. Johnston CC Jr, Slemenda CW, Melton LJ 3rd. Clinical use of bone densitometry. N Engl J Med. 1991. 324:1105–1109.
11. Mariette X, Khalifa P, Ravaud P, Frija J, Laval-Jeantet M, Chastang C, et al. Bone densitometry in patients with multiple myeloma. Am J Med. 1992. 93:595–598.
12. Karsh J. Diagnostic challenges in osteoporosis. Indications for bone densitometry and establishing secondary causes. Can Fam Physician. 2001. 47:1244–1250.
13. Mariette X, Bergot C, Ravaud P, Roux C, Laval-Jeantet M, Brouet JC, et al. Evolution of bone densitometry in patients with myeloma treated with conventional or intensive therapy. Cancer. 1995. 76:1559–1563.
14. Abildgaard N, Brixen K, Kristensen JE, Vejlgaard T, Charles P, Nielsen JL. Assessment of bone involvement in patients with multiple myeloma using bone densitometry. Eur J Haematol. 1996. 57:370–376.
15. Hanrahan CJ, Christensen CR, Crim JR. Current concepts in the evaluation of multiple myeloma with MR imaging and FDG PET/CT. Radiographics. 2010. 30:127–142.
16. Fechtner K, Hillengass J, Delorme S, Heiss C, Neben K, Goldschmidt H, et al. Staging monoclonal plasma cell disease: comparison of the Durie-Salmon and the Durie-Salmon PLUS staging systems. Radiology. 2010. 257:195–204.
17. Baur-Melnyk A, Buhmann S, Dürr HR, Reiser M. Role of MRI for the diagnosis and prognosis of multiple myeloma. Eur J Radiol. 2005. 55:56–63.
18. Moulopoulos LA, Varma DG, Dimopoulos MA, Leeds NE, Kim EE, Johnston DA, et al. Multiple myeloma: spinal MR imaging in patients with untreated newly diagnosed disease. Radiology. 1992. 185:833–840.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download