Abstract
Ependymomas usually occur in intraventricular or intraspinal locations. Intracranial extraaxial ependymomas (IEAEs) are very rare. Here, we present an unusual case of an IEAE in 25-year-old man. The patient experienced head trauma two and half years prior to presentation, at which time brain computed tomography (CT) showed only a dense calcification in the left ambient cistern. After two and a half years, follow up brain CT and magnetic resonance imaging depicted interval growth of a calcified mass with cystic change. The tumor was successfully treated surgically, and the pathologic examination confirmed ependymoma.
Ependymomas are slowly growing glial tumors that are generated from ependymal cells lining the ventricles of the brain and the central canal of the spinal cord. Ependymomas make up approximately 2-9% of intracranial gliomas and 30-60% of intramedullary spinal tumors (1). Ependymomas are a common neoplasm of the primary central nervous system in pediatric patients, but are rare in adults. Forty percent of intracranial ependymomas are supratentorial, while 60% are infratentorial in location (2). Intracranial extraaxial ependymomas (IEAEs) are extremely rare. We report a case of an IEAE in the left ambient cistern, which initially presented as a calcified mass.
A 25-year-old man was admitted with a headache that had persisted for 2 weeks. The neurologic examination showed an alert mental status with good coordination and normal reflexes. The patient had been in a car accident two and half years prior to presentation that resulted in head trauma. At that time, nonenhanced brain CT showed a 1.2 × 0.8 cm dense calcification in the left ambient cistern (Fig. 1A). There was no hydrocephalus, and this lesion was not connected to the cerebral aqueduct or fourth ventricle.
Two and half years later, follow up brain CT showed a predominantly cystic mass (2.5 × 2 cm) with fragmented calcifications in the left ambient cistern (Fig. 1B, C). The cystic component was located inferior to the fragmented calcification. On brain MRI, the cystic component of the mass demonstrated low signal intensity on the T1 weighted images and high signal intensity on the T2 weighted images. The midbrain was mildly compressed, but the right ambient cistern was relatively preserved. In the upper portion of the mass, T1 iso- to hyperintensity and T2 iso- to hypointensity were observed, which suggested solid components of the tumor. Superiorly, T2 weighted images showed hypointense areas that represented fragmented calcifications. Gadolinium-enhanced T1-weighted images showed heterogeneous enhancement in the solid component of the mass (Fig. 1D-G). There was no tentorial enhancement around the mass. On diffusion weighted images, there was no diffusion restriction in the cystic component of the mass. No significant tumor staining was noted on bilateral internal, external carotid, or vertebral artery angiograms.
The patient underwent surgical resection of the tumor with a left occipito-transtentorial approach. The tumor was soft and friable in texture and reddish in color. The tumor was mainly composed of the cystic mass. Calcifications were found in the upper portion of the tumor.
The histological diagnosis confirmed ependymoma. On hematoxylin and eosin staining, the tumor showed moderate cellularity with numerous calcifications. The tumor had a nuclear free-zone around a central blood vessel (pseudorosette) and sheets of spindled or epitheloid cells with round to oval nuclei (Fig. 1H, I). The tumor showed positive immunoreactivity for glial fibrillary acid protein (GFAP) and S-100 protein (Fig. 1J, K).
Postoperative follow up CT scans and MR images showed no evidence of residual tumor. The patient's symptoms regressed. He received adjuvant radiotherapy and chemotherapy after the pathologic diagnosis.
Ependymomas usually originate from ependymal cells in the cerebral ventricular surface and are most frequently located in the fourth ventricle (3). Extraaxial ependymomas are extremely rare. Several hypotheses have been proposed regarding the origins of IEAEs that are not related to ventricular ependymal cells, such as tumors arising from heterotopic ependymal cell residue in the subarachnoid space of the cerebellopontine angle (4), cavernous sinus (5), or on the trigeminal nerve (6) during fetal development that subsequently increase in size. Another hypothesis to explain supratentorial IEAEs is that tumors grow extraaxially from subcortical, subependymal residue, followed by necrosis and calcification of the originating subependymal residue, leaving an extraaxial ependymoma (7). Another hypothesis suggested that grossly nonvisible microscopic cellular tracts exist in development between the ventricle and extraaxial ependymoma that facilitate tumor extension into the subarachnoid space and then regress (8). Our case was a pure IEAE with no attachments to the midbrain or tentorium as observed intraoperatively, and probably originated from the heterotopic ependymal cell residue in the subarachnoid space.
The first case of IEAE was reported in 1976 (9) and to date, only 12 cases of IEAEs have been reported in the English literature. Locations of IEAEs were in the infratentorial cerebellar pontine angle (n = 5), supratentorial cerebral convexity (n = 5), and interhemispheric (n = 2). MR signal intensities of IEAEs are iso to low signal intensity on T1-weighted image and iso to high signal intensity on T2-weighted image. Most masses show heterogeneous enhancement. Preoperative diagnoses in previous reports were meningioma (n = 10) and schwannoma (n = 2). Our patient had a tumor located in the left ambient cistern, which initially presented as calcification only, which was not connected with the cerebral aqueduct. Additionally, there was no obstructive hydrocephalus. IEAE located in the ambient cistern is exceptional. Differential diagnoses of solid/cystic and well-circumscribed masses in the region of the ambient cistern include: trochlear nerve schwannoma, cystic meningioma arising from the tentorial edge, tentorial schwannoma, and exophytic glioma of the midbrain (10).
The histological features of ependymomas are perivascular pseudorosettes and ependymal rosettes. Ependymoma typically expresses GFAP, S-100 protein, and vimentin, and never expresses neuronal antigens in immunoreactivity. Meningioma and schwannoma do not express GFAP in immunoreactivity. In our case, immunohistochemistry studies of GFAP and S-100 protein were positive.
Supratentorial ependymomas are commonly cystic (40-85%), while infratentorial ependymomas are more often solid. The incidence of intratumoral calcification is about 50% of supratentorial ependymomas and 25-50% of infratentorial ependymomas (2). In our infratentorial case, only calcification was noted on initial nonenhanced brain CT, while fragmented calcification and cyst formation were seen on follow up CT and MR two and half years later.
To conclude, ependymoma may be considered when a predominantly cystic mass with multifocal calcification is observed in ambient cistern, although it is rare in this location.
Figures and Tables
Fig. 1
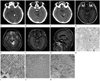
A 25-year-old man with an intracranial extraaxial ependymoma in left ambient cistern.
A. Nonenhanced CT shows a dense, large (1.2 × 0.8 cm) calcification in the left ambient cistern.
B, C. Nonenhanced CT shows a lobulated (2.5 × 2.0 cm) cystic mass (B) with fragmented calcifications (C) in the left ambient cistern.
D-G. An axial T1 weighted image shows a lobulated hypointense mass with a peripheral thin solid component in the left ambient cistern (D). An axial T2 weighted image shows a hyperintense mass with low signal multiple dot calcifications (E). Axial (F) and sagittal (G) gadolinium enhanced T1-weighted images show heterogeneous enhancement in the solid component of the mass.
H, I. A low-power photomicrograph (hematoxylin and eosin, × 40) shows that the tumor has moderate cellularity and numerous calcifications (H). A high-power photomicrograph (hematoxylin and eosin, × 400) shows sheets of spindled or ephithelioid cells with round to oval nuclei in the tumor. A nuclear free zone is seen around the central blood vessel (pseudorosette; white arrows) (I).
J, K. Immunohistochemical examination of the tumor reveals that S-100 (J) and glial fibrillary acid protein (K) stains are positive (× 200).
References
1. Rezai AR, Woo HH, Lee M, Cohen H, Zagzag D, Epstein FJ. Disseminated ependymomas of the central nervous system. J Neurosurg. 1996. 85:618–624.
2. Armington WG, Osborn AG, Cubberley DA, Harnsberger HR, Boyer R, Naidich TP, et al. Supratentorial ependymoma: CT appearance. Radiology. 1985. 157:367–372.
3. Choi JY, Chang KH, Yu IK, Kim KH, Kwon BJ, Han MH, et al. Intracranial and spinal ependymomas: review of MR images in 61 patients. Korean J Radiol. 2002. 3:219–228.
4. Fukui MB, Hogg JP, Martinez AJ. Extraaxial ependymoma of the posterior fossa. AJNR Am J Neuroradiol. 1997. 18:1179–1181.
5. Donich D, Lee JH, Prayson R. Giant extra-axial cerebellopontine angle/cavernous sinus ependymoma: case report. Neurosurgery. 1999. 44:195–198.
6. Little NS, Morgan MK, Eckstein RP. Primary ependymoma of a cranial nerve. Case report. J Neurosurg. 1994. 81:792–794.
7. Hayashi K, Tamura M, Shimozuru T, Kasamo S, Hirahara K, Kadota K, et al. Extra-axial ependymoma--case report. Neurol Med Chir (Tokyo). 1994. 34:295–299.
8. Lyons MK, Kelly PJ. Posterior fossa ependymomas: report of 30 cases and review of the literature. Neurosurgery. 1991. 28:659–664. discussion 664-665.
9. Hanchey RE, Stears JC, Lehman RA, Norenberg MD. Interhemispheric ependymoma mimicking falx meningioma. Case report. J Neurosurg. 1976. 45:108–112.
10. Ozawa N, Nakayama K, Ohata K, Okamura T, Inoue Y. Tentorial schwannoma: a case report. Br J Radiol. 2003. 76:421–424.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download