Abstract
Most distant metastases of dermatofibrosarcoma protuberans (DFSP) occurs with the repeated local recurrences and mainly in the lungs. Focusing on the imaging features, we report a unique occurrence of the distant metastasis of DFSP with fibrosarcomatous changes to the axillary soft tissue without previous local recurrences, possibly misdiagnosed as the accessory breast cancer.
Dermatofibrosarcoma protuberans (DFSP) is a rare low-grade malignant sarcoma that most commonly affects the dermis and the underlying subcutaneous soft tissues on the trunk and the proximal extremities (1-4). The incidence of DFSP is 0.8-4.2 cases per million persons per year and accounts for 2-6% of all soft tissue sarcomas (1-4). DFSP predominantly arises in young to middle-age males and is locally aggressive; and has a tendency of high-rate local recurrences (1, 4). As such, the primary treatment for DFSP is the complete surgical excision with the negative surgical margin (2-4). DFSP shows a low risk of metastasis accounting between 0.5% to 5%, which is always seen after the repeated local recurrences and almost always arises in the regional lymph nodes and the lungs (1-4). However, DFSP with fibrosarcomatous changes (DFSP-FS), a rare variant of DFSP, is considered to have a more aggressive clinical behavior resulting in a higher metastatic potential (5-8), although such is still debated (9).
Here, we present the radiologic findings of a rare case of axillary metastasis, focusing on the sonographic features of the metastasis mimicking accessory breast cancer in the abdominal DFSP-FS without local recurrences. To the best of our knowledge, this is the first report of the distant soft-tissue metastasis, other than in the lungs, in DFSP without local recurrences.
A 37-year-old woman was referred to our radiology department for the axillary sonography, who had undergone the wide excision for DFSP of the anterior abdomen 15 months ago. There was no evidence of distant metastasis in the previous chest and abdomen CT scans. She performed the routine postoperative follow-up study including the chest computed tomography (CT) (Sensation 16; Siemens Medical Solution, Erlangen, Germany). A round and solid nodule sized 2 cm, with mild homogenous enhancement, was incidentally observed in the left axilla on the chest CT scans (Fig. 1). Pulmonary metastasis was absent, and no locally recurred evidences were found on the CT scans. On the physical examination, there was no evidence of the local recurrence of DFSP in the abdomen. There was a focal bulging with a skin color change in the left axilla (Fig. 2). The bilateral breast and axillary ultrasound (US) (HDI 5000 or 3000, Philips-Advanced Technology Laboratories, Bothell, WA, USA) was performed. On the US, an irregular and hypoechoic mass with a hyperechoic rim, sized 2.1 × 1.9 cm, was seen at the left axillary subcutaneous fat layer (Fig. 3). There were no accompanied accessory breast tissues, abnormal lymph nodes or abnormal breast masses on the axillary and whole breast US. The most likely diagnosis was the accessory breast cancer arising in the axilla. However, considering the clinical history of DFSP, a lymph node or soft tissue metastasis from DFSP was included in the differential diagnosis. Subsequent mammography also revealed a round, hyperdense nodular density in the left axilla without other remarkable features (Fig. 4). The MRI (3.0 T, Signa Excite HDx, GE Healthcare, Milwaukee, WI, USA) demonstrated a solid tumor invading the skin and pectoralis major muscle at the left anterior axillary region. The mass showed iso-signal intensity on the T1 weighted images, central high signal intensity with peripheral low signal intensity on the T2 weighted images and peripheral enhancement on the post-contrast fat-suppressed T1 weighted images (Fig. 5). The US-guided 14-guage gun biopsy was performed, and the histopathologic features showed sarcomatous changes in most of the core specimens. The patient was referred to the general surgery department, and the mass was widely excised. The final pathologic results confirmed metastatic DFSP with fibrosarcomatous changes (Fig. 6) arising in the axillary subcutaneous soft tissue. The immunohistochemistry showed positive SMA, negative Desmin, CD34, EMA, C-kit, equivocal MIC2 (CD99) and mildly increased Ki-67 (proliferative index). It was almost similar to the primary tumor of the abdominal skin. She survived for 7 months without recurrences after the surgical excision of the metastasis.
The definite diagnosis of the typical DFSP requires histology showing a prominent storiform pattern of the monomorphic fibroblast-like cells that invade into subcutis arising in the dermis on incisional or the excisional biopsy (1, 4). When DFSP shows high-grade fibrosarcomatous changes (spindle cells intersecting at acute angles, coarser chromatin, increased mitotic activity) in more than 5% of the tumor tissues, it is classified as DFSP-FS (6). Although there is still a controversy on the prognostic influence of the DFSP-FS (9), many reports have suggested that it is associated with a worse prognosis (5-8). Mentzel et al. (7) studied 41 patients with DFSP-FS and concluded that the fibrosarcomatous changes in DFSP represented a form of tumor progression in DFSP, and was associated with a significantly more aggressive clinical course than the ordinary DFSP.
Although DFSP-FS is considered to have a higher risk of metastasis, there are currently no published reports on the distant metastasis other than that in the lungs. Our case was exceptional and unique, as the distant metastasis of DFSP-FS occurred in the axillary soft tissue, particularly, without the local recurrences of the primary DFSP.
When DFSP is diagnosed, an extensive staging workup is not routinely indicated, but an assessment of the regional lymph node and the pulmonary metastases is preoperatively needed. The postoperative follow-up chest CT is indicated in patients with prolonged, locally advanced or recurrent DFSP or in patients with DFSP-FS.
The imaging features of DFSP have been reported scarcely in few articles (10-13). Imaging studies are not routinely performed for DFSP, because its diagnosis is made on the clinical appearance and the superficial biopsy.
However, US is a simple imaging tool to apply for the superficially growing DFSP. In this case, US was the first choice of the diagnostic method for the evaluation of the axillary mass after the incidental detection of the chest CT scans. The US could be used to guide for the real-time interventional core biopsy approaches, and the 14-guage core biopsy specimens histologically depicted the sarcomatous changes.
Shin et al. (12) studied the sonographic findings of DFSP located in the back of 4 patients. All cases were oval masses in the subcutaneous layer that were abutting against the skin, showing a focal lobulated margin with hypoechogenicity or an irregular margin with mixed echogenicity (12). According to a few case reports of DFSP of the breast (11), the masses were also oval and circumscribed, hypoechoic or mixed-echogenic on the US. The primary DFSP of our case was concordant with the sonographic findings in the previous studies. However, our axillary DFSP exhibited the sonographic features of an irregular and poorly defined hypoechoic mass with the hyperechoic rim only involving the subcutaneous fat tissue, which was first differentiated from the axillary accessory breast cancer.
For this axillary mass, the preoperative MR imaging demonstrated a solid tumor invading the skin and the pectoralis major muscle at the left anterior axillary region. It was a non-specific, axillary soft tissue tumor invading the skin and muscles on the MR imaging, which corresponded with the previous studies of MR imaging (13). Torreggiani et al. (13) reported that MR imaging allowed for the accurate preoperative assessment and aided in the diagnosis of atypical or difficult cases of DFSP.
CT is not indicated except in the suspected underlying bone involvement. In this case, the chest CT was done for the evaluation of pulmonary metastases, and the axillary mass was incidentally discovered. The mass was non-specific, round, isodense and solid nodule with mild homogenous enhancement on the CT scans.
Immunohistochemically, CD34 is one of the most useful stains to differentiate DFSP from other soft tissue tumors (1, 2). The sensitivity of CD34 staining in DFSP ranges from 84 to 100%, whereas DFSP-FS is CD34 positive in about half of the cases (8). In this case, the histologic findings showed significant areas of the high-grade sarcomatous changes in both the primary and the metastatic lesions of DFSP. The CD34 was negative or weakly positive in both.
DFSP should be treated with a wide surgical excision, and the Mohs micrographic surgery or the Imatinib use has been studied as a substitute treatment (1).
In conclusion, although the distant metastasis of DFSP almost always arises in the lungs with repeated local recurrences, it may occur in the axillary soft tissue, mimicking the accessory breast cancer, without local recurrences. The knowledge of the clinical and imaging features of our case would be helpful for the radiologists in accurately diagnosing DFSP.
Figures and Tables
Fig. 1
A postoperative routine chest CT axial image revealed a 2 cm sized round and solid nodule with mild homogenous enhancement in the left axilla (arrow). Pulmonary metastasis was absent and no locally recurred evidences were found on chest and abdominal CT scans.
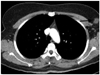
Fig. 2
Physical examination showed postoperative scar in the left abdominal skin and focal bulding with a blue skin color change in the left axilla (arrow).
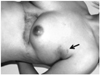
Fig. 3
A 2.1 × 1.9 cm sized irregular and hypoechoic mass with a hyperechoic rim (arrows) was seen at the left axillary subcutaneous fat layer on ultrasound.
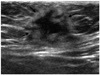
Fig. 4
Mammography showed a round and hyperdense nodular density in the left axilla (arrow) without other remarkable features.
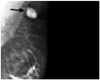
Fig. 5
A post-contrast fat-suppressed T1 weighted MR axial image demonstrated a peripherally enhancing solid tumor with internal necrosis invading the skin and the pectoralis major muscle at the left anterior axillary region (arrows).
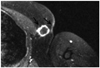
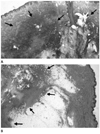
Fig. 6
The final pathologic results confirmed metastatic dermato fibrosarcoma protuberans with fibrosarcomatous changes.
A. Pathologic specimen of an initial abdominal lesion showed sarcomatous changes (arrows) in dermatofibrosarcoma protuberans arising in the dermis (hematoxylin-eosin, × 12).
B. Pathologic specimen of a recurrent axillary lesion showed similar histologic freatures of sarcomatous changes in dermatofibrosarcoma protuberans (arrows) locating in subcutaneous fatty layer (hematoxylin-eosin, × 12).
References
1. Bogucki B, Neuhaus I, Hurst EA. Dermatofibrosarcoma Protuberans: A Review of the Literature. Dermatol Surg. 2012. [Epub ahead of print].
2. Bowne WB, Antonescu CR, Leung DH, Katz SC, Hawkins WG, Woodruff JM, et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000. 88:2711–2720.
3. Erdem O, Wyatt AJ, Lin E, Wang X, Prieto VG. Dermatofibrosarcoma protuberans treated with wide local excision and followed at a cancer hospital: prognostic significance of clinicopathologic variables. Am J Dermatopathol. 2012. 34:24–34.
4. Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004. 101:2503–2508.
5. Abbott JJ, Oliveira AM, Nascimento AG. The prognostic significance of fibrosarcomatous transformation in dermatofibrosarcoma protuberans. Am J Surg Pathol. 2006. 30:436–443.
6. Ding J, Hashimoto H, Enjoji M. Dermatofibrosarcoma protuberans with fibrosarcomatous areas. A clinicopathologic study of nine cases and a comparison with allied tumors. Cancer. 1989. 64:721–729.
7. Mentzel T, Beham A, Katenkamp D, Dei Tos AP, Fletcher CD. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998. 22:576–587.
8. Palmerini E, Gambarotti M, Staals EL, Zanella L, Sieberova G, Longhi A, et al. Fibrosarcomatous changes and expression of CD34+ and apolipoprotein-D in dermatofibrosarcoma protuberans. Clin Sarcoma Res. 2012. 2:4.
9. Goldblum JR, Reith JD, Weiss SW. Sarcomas arising in dermatofibrosarcoma protuberans: a reappraisal of biologic behavior in eighteen cases treated by wide local excision with extended clinical follow up. Am J Surg Pathol. 2000. 24:1125–1130.
10. Kransdorf MJ, Meis-Kindblom JM. Dermatofibrosarcoma protuberans: radiologic appearance. AJR Am J Roentgenol. 1994. 163:391–394.
11. Lee SJ, Mahoney MC, Shaughnessy E. Dermatofibrosarcoma protuberans of the breast: imaging features and re view of the literature. AJR Am J Roentgenol. 2009. 193:W64–W69.
12. Shin YR, Kim JY, Sung MS, Jung JH. Sonographic findings of dermatofibrosarcoma protuberans with pathologic correlation. J Ultrasound Med. 2008. 27:269–274.
13. Torreggiani WC, Al-Ismail K, Munk PL, Nicolaou S, O'Connell JX, Knowling MA. Dermatofibrosarcoma protuberans: MR imaging features. AJR Am J Roentgenol. 2002. 178:989–993.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download