Abstract
Nasopharyngeal masses are usually malignant, and benign nasopharyngeal tumors such as hemangioma are unusual. In adults, hemangiomas do not involute spontaneously, but progress. Imaging modalities are useful to rule out other malignancies and vascular lesions and to evaluate the lesion. Most hemangiomas require no therapy, but certain factors such as age of the patient and location and size of the lesion may make treatment necessary. We report a case of an unusual nasopharyngeal hemangioma treated with endoscopic excision in an adult who complained of hearing loss.
Hemangiomas are the most common vascular lesions of infancy. More than half of hemangiomas affect the head and neck, mainly in the oropharynx and rarely in the nasopharynx (1). Unlike infantile hemangiomas, adult hemangiomas progressively enlarge and do not spontaneously regress and they are to be neoplastic though (2). Most nasopharyngeal tumors are malignant (3). Infantile hemangiomas are usually diagnosed by clinical presentation and physical examination, and do not require treatment. However, a hemangioma in an adult warrants further evaluation and treatment. Here, we report a case of a 45-year-old male patient with a benign hemangioma involving the nasopharynx.
A 45-year-old man presented with intermittent left nasal obstruction of about 6 months' duration and admitted left hearing difficulty for a week. He had no significant medical history and was a 20-pack-year smoker. Nasal endoscopy showed a protruding, reddish lesion in the left nasal cavity. Contrast-enhanced neck computerized tomography (CT) demonstrated an oval, well-defined, heterogeneous mass that was isodense to hypodense compared with adjacent muscle; the mass measured 3.0 × 3.5 × 5.9 cm in the left nasopharynx and extended into the left oropharynx. After contrast administration, the mass showed heterogeneous enhancement with enhanced tubular structures (Fig. 1A-C). The patient had left otomastoiditis secondary to left nasopharyngeal mass, but the neck lymph nodes were not enlarged.
Magnetic resonance imaging (MRI) was performed to further evaluate the tumor. The mass was a relatively well-defined soft tissue mass located in the left nasopharynx. It showed iso-signal intensity compared with adjacent muscle in T1-weighted images and slightly high-signal intensity in T2-weighted images, and was intensely enhanced after contrast administration (Fig. 1D-F). It contained signal-void tubular structures thought to be vessels, and there was secondary fluid collection in the left middle ear cavity and mastoid air cells, suggestive of left otomastoiditis. The labyrinthine and intracanalicular segments of the right facial nerve showed intense enhancement, compatible with right facial neuritis. The hypervascular mass in the left nasopharynx was thought to be either a nasopharyngeal carcinoma or a benign tumor such as a hemangiopericytoma, hemangioendothelioma, angiofibroma, or other vascular malformation.
With the left nasal cavity obstructed and the hearing difficulty due to left otomastoiditis, complete removal of the lesion was necessary. Transfemoral cerebral angiography showed that the lesion was supplied by multiple branches of the left internal maxillary, ascending pharyngeal, and other branches of the external carotid artery (Fig. 1G, H). Intense tumor staining with early venous drainage was noted. Before excision, embolization was required to prevent massive bleeding from tumor vessels. Embolization was performed using polyvinyl alcohol. After embolization, the patient underwent endoscopic excision and biopsy of the tumor. At excision, the tumor was confined in submucosal space of the nasopharynx and nasopharyngeal mucosa and prevertebral fascia were intact.
Histopathology of the tumor showed prominent, variably dilated, thick-walled vessels and some small capillaries arranged in lobules (Fig. 1I). There was no endothelial atypia or fibrovascular stroma, consistent with a mixed hemangioma.
On day 4 after endoscopic excision, both nasal packings were removed, and the patient was discharged without complications 7 days after surgery
Hemangiomas are benign vascular tumors. They occur most commonly in infancy and involve mainly the head and neck (1, 4). Nasopharyngeal hemangiomas are rare and therefore less extensively studied (1, 5, 6). Benign tumors of the nasopharynx, including juvenile angiofibroma, hemangioma, hemangiopericytoma, and Kimura's disease, are uncommon (3).
There are several classifications of vascular anomalies. In 1982, Mulliken and Glowacki (7) introduced two categories of vascular anomalies in infancy, based on clinical history and histological characteristics: hemangioma and vascular malformation.
Hemangiomas are not congenital, but grow quickly by endothelial proliferation in the proliferative stage and then regress slowly in the involution stage. In contrast, vascular malformations are congenital structural anomalies and grow with normal endothelial turnover. It is sometimes difficult to differentiate high-flow lesions of vascular malformations from hemangiomas, and some types of hemangiomas (e.g., capillary hemangiomas and cavernous hemangiomas) progress, rather than regress or involute. However, most hemangiomas can be differentiated from vascular malformations based on clinical findings and physical examination. Controversy exists concerning the nomenclature of hemangiomas in adults, which are far less common than infantile hemangiomas. So-called "adult hemangiomas" enlarge with age and do not involute spontaneously; thus, they are thought to be more neoplastic than infantile hemangiomas (2). Hemangiomas occur more frequently in females and Caucasians, and are usually small and isolated lesions. Although the lesion may be reddish, pinkish, or bluish in accordance with its depth and location, hemangiomas are generally firm, bright red, pulsatile, and non-compressible compared with vascular malformations (2).
There are some complications associated with hemangiomas in the head and neck. These include obstruction of the visual tract or subglottic area, mild-to-moderate hearing loss (as in our case), ulceration, bleeding, and even life-threatening complications such as coagulopathies (Kasabach-Merritt syndrome), congestive heart failure, and airway obstruction (5, 6). Most infantile hemangiomas in the head and neck involute spontaneously, whereas adult lesions do not. However, several studies have reported that approximately 40% of children with hemangiomas require further treatment due to complications (8). Treatment of a hemangioma depends on the age of the patient and location and size of the lesion (5). Although corticosteroids have been used as first-line therapy of hemangiomas, they are currently used only for life-threatening conditions. In many recent studies, propranolol was proposed for initial conservative therapy. Propranolol is effective in reducing tumor size, but its mechanism is unclear (8). Laser excision, sclerotherapy, and intralesional steroid treatment can be considered as alternative treatment options (2, 8). Surgical excision is the most effective treatment and often results in a complete cure. In the current case, where the patient had inflammation of left middle ear cavity with hearing loss related to the tumor, we performed transnasal endoscopic excision, which is minimally invasive and reduces surgery-related complications.
For precise diagnosis, ultrasonography (including Doppler flow studies), CT, MRI, and sometimes angiography are used. Nasal endoscopy is useful in cases of nasopharyngeal hemangioma, as the lesion is inaccessible to physical examination. However, it can visualize only the focal portion of the lesion, often necessitating imaging studies such as CT and MRI for a full evaluation (3, 9). On MRI, the lesion appears as a lobulated, soft-tissue mass with multiple prominent vascular elements and intermediate signal intensity on T1-weighted images, moderately high signal intensity on T2-weighted images, and intense enhancement (9, 10). Hemangiomas are commonly heterogeneous, although homogeneous signal intensity is observed with high perfusion in small-capillary hemangiomas and low perfusion in cavernous hemangiomas (9). Flow voids are seen within high-flow feeding arteries and draining veins on spin-echo (SE) images without an arteriovenous shunt (4). On unenhanced CT images, hemangiomas show attenuation similar to that of muscle. CT with contrast enhancement is useful in vascular lesions and other malignancies. Hemangiomas appear as well-circumscribed, densely enhanced lesions with "rapid filling-in", correlated with high-flow lesions. Involuting hemangiomas shrink and present an increasing fibrofatty matrix with decreased vascularity and relative enhancement (10). Angiography is useful for observing vascular anomalies, and it allows selective embolization to be performed for reducing intraoperative bleeding (2).
In our case, we differentiated the lesion from a hemangiopericytoma, hemangioendothelioma, and angiofibroma. Hemangiopericytomas and hemangioendotheliomas are malignant vascular tumors and are intermediately aggressive (2). Hemangiopericytomas are rare and usually involve the lower extremities, retroperitoneum, and pelvis. About 15% of these tumors occur in the head and neck region, and rarely in the pharynx (1). Angiofibromas arise in the region of the sphenopalatine foramen and extend to the pterygopalatine fossa (3). They are highly vascular tumors, with nonspecific imaging findings that are similar to those of hemangiomas. CT reveals moderate to markedly enhanced masses, and MRI demonstrates lesions with prominent vascular structures and with intermediate signal intensity on T1-weighted images and hyperintensity on T2-weighted images (9). Some lesions, including sarcomas, hemanigopericytomas, and fibrosarcomas, show perilesional edema, whereas hemangiomas do not (4). Flow voids vessels may be high-flow feeding arteries and draining veins on SE images and high signal intensity on gradient echo images, and showed early intense enhancement on contrast-enhancement images. In proliferative stage of hemangiomas, an arteriovenous shunt is not seen, unlike arteriovenous malformations as high-flow vascular malformations (4). It is necessary to differentiate from nasopharyngeal carcinomas, the most common primary malignancy in the nasopharynx (3). Nasopharyngeal carcinomas show predominantly focal or diffuse nasopharyngeal mucosal thickening and cervical lymphadenopathy. As their nature is aggressive, they can demonstrate perineural spread, parapharyngeal space or bone marrow involvement and involve the adjacent paranasal sinuses and intracranial extension. On MR imaging, the lesions show hypointense to isointense signal intensity on T1-weighted images and hypointense signal intensity on T2-weighted images, and show moderate to intense enhancement on contrast-enhanced images. Therefore, compared with nasopharyngeal carcinomas, nasopharyngeal hemangiomas are intact of mucosa and less frequently involve cervical lymph nodes and adjacent structures.
In conclusion, we report here an unusual case of hemangioma, which mimicked a malignant tumor or vascular malformation on CT and MRI, in the nasopharynx in an adult. Although rare, a hemangioma should be considered in the differential diagnoses of a well-circumscribed, solid nasopharyngeal mass with hypervascularity.
Figures and Tables
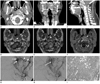 | Fig. 1CT, MR, angiography, and pathologic findings of nasopharyngeal hemangioma in a 45-year-old man.
A-C. Contrast-enhanced neck CT scan (A. axial, B. coronal, C. sagittal image) shows an oval, well-defined, heterogeneously enhanced mass (arrows) with tubular enhanced structures (arrowheads) in the nasopharynx and extending into the oropharynx.
D. A T1-weighted image shows an isointense mass (arrows) with inner signal-void tubular structures (arrowhead) in the left nasopharynx.
E. An axial FSE T2-weighted image shows a heterogeneous mass (arrow) with slightly high signal intensity and an inner signal-void tubular lesion (arrowhead).
F. A contrast-enhanced 3D-SPGR image shows an intensely enhanced mass (arrows) in the left nasopharynx.
G, H. Selective left external carotid artery angiography with left anterior oblique view shows hypervascular tumor staining (arrows), supplies from posterior superior alveolar artery, arising from the internal maxillary artery (arrowheads) in the early arterial phase, and an early draining vein (long arrow) in the late arterial phase.
I. The tumor consists of variously sized, thick-walled vessels. In some parts, there is lobular growth of small capillary-sized vessels with central feeding vessels (H&E, × 40).
Note.-FSE = fast spin echo, 3D-SPGR = 3 dimensional spoiled gradient echo
|
References
1. Som PM, Curtin HD. Head and neck imaging. 2011. 5th ed. St. Louis, MO: Mosby;1749–1779.
2. Connor SE, Flis C, Langdon JD. Vascular masses of the head and neck. Clin Radiol. 2005. 60:856–868.
3. Chong VF, Fan YF. Radiology of the nasopharynx: pictorial essay. Australas Radiol. 2000. 44:5–13.
4. Flors L, Leiva-Salinas C, Maged IM, Norton PT, Matsumoto AH, Angle JF, et al. MR imaging of soft-tissue vascular malformations: diagnosis, classification, and therapy follow-up. Radiographics. 2011. 31:1321–1340. discussion 1340-1341.
5. Ramaligam K, Ramalingam R, Rayappa C, Chaudhary A. Giant haemangioma of the naso-orohypopharynx treated by midline mandible split. Bangladesh J Otorhinolaryngol. 2011. 17:62–67.
6. Strauss M, Widome MD, Roland PS. Nasopharyngeal hemangioma causing airway obstruction in infancy. Laryngoscope. 1981. 91:1365–1368.
7. Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982. 69:412–422.
8. Fuchsmann C, Quintal MC, Giguere C, Ayari-Khalfallah S, Guibaud L, Powell J, et al. Propranolol as first-line treatment of head and neck hemangiomas. Arch Otolaryngol Head Neck Surg. 2011. 137:471–478.
9. Vilanova JC, Barceló J, Smirniotopoulos JG, Pérez-Andrés R, Villalón M, Miró J, et al. Hemangioma from head to toe: MR imaging with pathologic correlation. Radiographics. 2004. 24:367–385.
10. Razek AA, Huang BY. Soft tissue tumors of the head and neck: imaging-based review of the WHO classification. Radiographics. 2011. 31:1923–1954.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download