Abstract
White matter hyper intensities (WMHI) on MRI are not rare in patients with progressive systemic sclerosis (PSS). In this presentation, WMHI were developed in both middle cerebellar peduncles and temporal white matter in a patient with PSS, and regressed after medication of high dose steroid. However, new lesions were developed in the subcortices of both precentral gyri, and progressed rapidly to tumefactive hyperintensity on MRI. We report an unusual relapsing and progressive tumefactive demyelinating form of central nervous system involvement in PSS.
The central nervous system (CNS) involvement in progressive systemic sclerosis (PSS) is rare, which has been considered to be either uncommon or a secondary consequence of hypertension, uremia, pulmonary dysfunction and steroid treatment (1-3). White matter hyper intensities (WMHI) on MRI were more common in patients with PSS than in the control group, which might be asymptomatic and inadequate of the disease duration (2, 3).
We report a unique case of PSS where WMHI have arisen in both the middle cerebellar peduncles and left temporal lobe, and made rapid progress into tumefactive WMHI in the subcortices of both precentral gyri. To the best of our knowledge, tumefactive form of PSS has not been reported.
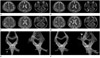
A 68-year-old man was admitted to our hospital with the onset of dysarthria for 1 month. Vocal cord movement was slightly impaired, but not fixed. Neurologic examination was normal, except moderate dysarthria. He was alert and normotensive. T2 weighted image (T2-WI) of MRI showed multiple small WMHI in the left pons and both cerebral white matters (Fig. 1A). On past history, the patient had presented peripheral type of the left facial palsy at age 26. He had 4-year-history of Raynaud phenomenon and 3-year-history of skin thickening and hyperpigmentation. One year ago, he was diagnosed as PSS, limited cutaneous type.
Two months later, dysarthria has been progressed, with impairment of vibration perception in both legs and gait disturbance was developed. T2-WI of MRI revealed WMHI in both middle cerebellar peduncles and left temporal lobe (Fig. 1B), and increased diffusivity on diffusion weighted MR image (DWI). MR spectroscopy showed decrease in the NAA peak at 2.0 ppm. MR angiography (MRA) for evaluation of the possible cerebral macroangiopathy showed to be normal. No demonstrable enhancement was found in contrast to an enhanced MR imaging. Diffusion tensor MRI showed decreased fiber connectivities in the right cerebellar peduncle (Fig. 1E, F).
Six months later, the WMHI of bilateral middle cerebellar peduncles and left temporal lobe were regressed, but patchy WMHI were developed in the subcortices of both precentral gyri (Fig. 1C); those lesions were progressed into tumefactive WMHI after one month (Fig. 1D) and showed increased diffusivity on diffusion weighted image. The patient was deteriorated gradually.
PSS is a multisystem connective tissue disease characterized by proliferation of the vascular tissue, obliterative microvascular lesions and diffuse organ fibrosis. Gastrointestinal tract, lungs, heart and kidneys are mostly affected. CNS involvement in PSS is rare, possibly due to paucity of connective tissue and lack of external lamina, with sparse media and adventitia (1, 4, 5). Cerebral hypoperfusion, suggestive of impaired quantitative microcirculation, was observed in PSS patients (6). Reduction of regional cerebral blood flow might be related to PSS, although the vast majority of patients remained in subclinical phase. In PSS, the primary site of vascular involvement is at the level of small arteries and capillaries. However, several authors have reported CNS abnormalities related with carotid and intracranial vasculitis in patients with PSS (7-9).
Most common MR findings of CNS involvement of PSS is WMHI, which are thought to result from ischemic vasculopathy, supporting the hypothesis of early and frequent brain involvement in patients with PSS. In this case, WMHI of both middle cerebellar peduncles and left temporal lobe that showed at the first presentation were thought as ischemic vasculopathy as the usual complication of PSS. The lesions regressed after medication of high dose steroid. However, rapid progression was demonstrated later into tumefactive hyper intensities in the subcortices of both precentral gyri, which has not been described as CNS involvement of PSS. MRA for evaluation of the possible cerebral macroangiopathy showed normal. Diffusion tensor MRI showed decreased fiber connectivities in the right cerebellar peduncle and the subcortex of the right precentral gyrus. These lesions could not be explained as resulting from ischemic vasculopathy or as a form of microangiopathy. In our opinion, white matter change might be caused by a localized autoimmune demyelinating disease. The evidence supporting this hypothesis is: A) an apparent good response to high-dose corticosteroid treatment as suggested by the clinical improvement at first presentation; B) no steno-occlusive lesion was detected on MRA; C) no vascular risk factor was present, which was documented during the hospitalisation; D) echocardiography was unable to detect embolic sources; and E) increased diffusivity of the lesion was detected on DWI, which considered vasogenic edema, as result of demyelination, rather than acute ischemic lesion.
When one identifies multifocal relapsing and remitting WMHI, the differential diagnosis may be worth the consideration: multiple sclerosis, autoimmune disease, such as behcet's disease, acute disseminated encephalomyelitis or lymphoma. PSS is a multisystem disease primarily affecting the skin, but may involve other organs including lung and kidney. Only a few cases of coexistence of PSS and multiple sclerosis have been described. Since several connective tissue diseases are associated with transverse myelitis, including systemic lupus erythematosus and Sjögren's syndrome, there are discussions whether the transverse myelitis in patients with PSS were an independent overlapping event or a manifestation of PSS (10).
In summary, we present the first report of relapsing and progressive tumefactive demyelinating form in a patient with PSS probably due to demyelination of white matter. Conventional MRI was very useful to detect the CNS lesions, and diffusion tensor imaging and MR spectroscopy could be helpful to evaluate the severity and the change of white matter involvement.
Figures and Tables
Fig. 1
A 68-year-old man with central nervous system involvement of progressive systemic sclerosis.
A. Axial T2-weighted image shows multiple small white matter hyperintensities in the left pons and cerebral white matter of both hemispheres.
B. Follow-up MRI obtained 2 months later shows white matter hyperintensities in both cerebellar peduncles (arrow) and left temporal lobe.
C. Follow-up MRI obtained 6 months later shows regression of the white matter hyperintensities in both cerebellar peduncles; however, newly developed patchy white matter hyperintensities are seen in the subcorticies of both precentral gyri (arrow).
D. Lesions were progressed into tumefactive white matter hyperintensities after one month (arrow).
E. Diffusion tensor MRI showing decreased fiber connectivities in the right cerebellar peduncle (arrow).
F. Follow-up imaging shows decreased white matter fiber tract in the right frontal lobe (double arrows).
References
1. Lucivero V, Mezzapesa DM, Petruzzellis M, Carella A, Lamberti P, Federico F. Ischaemic stroke in progressive systemic sclerosis. Neurol Sci. 2004. 25:230–233.
2. Mohammed RH, Sabry YY, Nasef AA. Brain MRI screening showing evidences of early central nervous system involvement in patients with systemic sclerosis. Rheumatol Int. 2011. 31:667–671.
3. Sardanelli F, Iozzelli A, Cotticelli B, Losacco C, Cutolo M, Sulli A, et al. White matter hyperintensities on brain magnetic resonance in systemic sclerosis. Ann Rheum Dis. 2005. 64:777–779.
4. Kanzato N, Matsuzaki T, Komine Y, Saito M, Saito A, Yoshio T, et al. Localized scleroderma associated with progressing ischemic stroke. J Neurol Sci. 1999. 163:86–89.
5. Simeoni S, Puccetti A, Tinazzi E, Tomelleri G, Corrocher R, Lunardi C. Systemic sclerosis and superficial siderosis of the central nervous system: casuality or causality? Rheumatol Int. 2008. 28:815–818.
6. Nobili F, Cutolo M, Sulli A, Castaldi A, Sardanelli F, Accardo S, et al. Impaired quantitative cerebral blood flow in scleroderma patients. J Neurol Sci. 1997. 152:63–71.
7. Chung MH, Sum J, Morrell MJ, Horoupian DS. Intracerebral involvement in scleroderma en coup de sabre: report of a case with neuropathologic findings. Ann Neurol. 1995. 37:679–681.
8. Stone J, Franks AJ, Guthrie JA, Johnson MH. Scleroderma "en coup de sabre": pathological evidence of intracerebral inflammation. J Neurol Neurosurg Psychiatry. 2001. 70:382–385.
9. Pathak R, Gabor AJ. Scleroderma and central nervous system vasculitis. Stroke. 1991. 22:410–413.
10. Torabi AM, Patel RK, Wolfe GI, Hughes CS, Mendelsohn DB, Trivedi JR. Transverse myelitis in systemic sclerosis. Arch Neurol. 2004. 61:126–128.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download