Abstract
Intraductal papillary mucinous neoplasm (IPMN) is known to arise from intraductal proliferation of mucinous cells with findings of marked dilatation of the biliary or pancreatic duct. There are reports of the metastasis and extension of pancreatic IPMN. However, cases of biliary IPMN with direct metastasis, or metastasis to distant locations, are rare. We present a case of metastasis of biliary IPMN with unexpected direct extension into the thoracic cavity, and we attempt to account for the mechanism of this extension.
Intraductal papillary mucinous neoplasms (IPMN) involve the intraductal proliferation of mucinous cells that are arranged in a papillary pattern with increasing frequency (123). Recently, the existence of pancreatic IPMN, although rare, is well-established. However, there are few reports of biliary IPMN (1), and to our knowledge, there are no published reports of its direct metastasis and extension. We present a case of metastasis of biliary IPMN that unexpectedly extended directly into the thoracic cavity, and we attempt to account for this extension.
An 82-year-old woman visited our emergency room presenting with a one-month cough and fever. She had a history of cholecystectomy due to gallstones and treatment for acute myocardial infarction, diabetes and hypertension; otherwise, she had no other underlying diseases.
On arrival, vital signs were stable, but physical examination revealed reduced sounds of breathing in the left lower lung field with rale. On a chest plain radiography, a consolidative lesion was noted in the left lower lung field. Multidetector computed tomography was scheduled for further work up, with under the impression of pneumonia. This showed dilatation of the common bile duct and marked disproportional dilatation of the left intrahepatic duct, with a maximum diameter of 2.0 cm; however, there was no evidence of any intraductal mural nodule. There was also no definite evidence of a mass in the proximal intrahepatic bile duct, or of lymph node enlargement. The findings strongly suggested biliary IPMN, with dilated bile duct extending to the diaphragm, the result of communication with the left thoracic space and leading to empyema in the left thorax (Fig. 1A-C).
For further evaluation, percutaneous transhepatic cholangiography was done. Cholangiography showed a marked dilatation of the left intrahepatic duct and communication with left thoracic space (Fig. 1D). Surgical operation for the biliary IPMN and empyema in the left thorax was strongly recommended. However, the patient declined a left lobectomy of the liver, on account of her age, but underwent an operation for the lung empyema.
In the operation field, severe membranous adhesions were noted over the entire thoracic cavity; in addition, a loculated effusion filled with a mucinous substance, which had also entered the pleural cavity, could be seen, together with lung abscesses. On histopathologic examination of the thoracic cavity, metastatic adenocarcinoma was confirmed. Although the lesion in the liver was not histopathologically confirmed, the mucinous substance in the thoracic cavity with severe adhesions extending from the liver via a diaphragm suggests metastasis with direct extension.
Some papillary tumors of the bile duct secrete an excessive amount of mucin, which may disturb bile flow and cause severe ductal dilatation. There are few reports of mucin-hyper-secreting bile duct tumors (3) or of the metastasis and extension of biliary IPMN. Biliary IPMN has been described as the counterpart of pancreatic IPMN, and has striking similarities to it in terms of histopathologic features, production of large amounts of mucin, pathophysiology, and clinical manifestations (124).
Pancreatic and biliary IPMN are low-grade malignancies that are generally limited to the mucosa, although, they may invade the ductal wall in the late phase; they can be classified as displaying adenoma, borderline, and carcinoma-like adenoma-carcinoma sequences (13). Lim et al. (2) reported that, like pancreatic IPMN, biliary IPMN is often a benign biliary mucin- producing lesion, manifesting as a biliary papillary hyperplasia, dysplasia, or adenoma. Further, in a recent study, Yeh et al. (5) presented a cholangiographic classification of biliary IPMN from benign to invasively malignant, following the course of chronic inflammation, dysplasia and carcinoma in situ to invasive carcinoma.
There are a few reports of deep invasion and nodal metastasis of pancreatic IPMN (6). There are examples of different growth patterns, including invasive growth and fistulous extension to adjacent organs (7), and Shibahara et al. (8) have described a lymph node metastasis and lymphatic, venous and perineural invasion of biliary IPMN.
In imaging studies of IPMN, papillary tumors can be detected by ultrasound, computed tomography, endoscopic retrograde cholangiopancreatography or tube cholangiography. However, small, sessile tumors that spread along the mucosal surface may be difficult or impossible to detect. Moreover, since the attenuation of mucin in a computed tomography and its signal in magnetic resonance imaging, are the same as that of the water, it is difficult to differentiate the mucinous component from the water or other contents. However, due to the overproduction of mucin in IPMN, the biliary tree can become diffusely dilated, and, when a tumor develops in the lobar or segmental duct, as in the present case, the duct involved can dilate disproportionately than the normal duct (3).
The appearance of IPMN depends on two factors; epithelial proliferation and mucin secretion; a papillary mass will result when epithelial proliferation predominates. On the other hand, when mucin secretion predominates, the bile duct will fill with mucin, and gross dilatation will result, without definite evidence of papillary tumor or intraductal mural nodule, as in the present case (9).
Kaye (10) has discussed various explanations for the pleuropulmonary complications in pancreatitis. In cases of acute pancreatitis, pleuropulmonary complications could be caused by direct contact or hematogenous carriage of pancreatic enzymes. Other possible mechanisms are the direct fluid movements via a natural hiatus or transfer of fluid into the trans-diaphragmatic lymphatics. The latter could account for the direct extension of the biliary IPMN to the pleural cavity (10).
As explained earlier, there was an article concerning the lymphatic invasion of the biliary IPMN (8). It could be a possible explanation of transfer of fluid into pleural cavity by transdiaphragmatic lymphatics, like in acute pancreatitis in our case. Early work established the existence of numerous lymphatic vessels, joining the lymphatic networks of the superior and inferior diaphragmatic surface (10). Hence, we could hypothesize that the biliary IPMN could metastasize via this route to the lymphatics and could extend to the thoracic cavity via the extensive network of transdiaphragmatic lymphatics.
In our case, radiologically diagnosed biliary IPMN was connected to the left lower thoracic space. Although there was no definite pathologic confirmation of the hepatic lesion, mucinous material was noted on the surgical field, and the finding of metastatic cells in the pleura was highly suggestive of the biliary IPMN with direct metastasis to the thoracic cavity. IPMN, including the biliary IPMN, is thought to be a low-grade malignancy and few cases of metastasis or extension of biliary IPMN have been reported. Nevertheless, our case shows that the biliary IPMN can metastasize, despite its low-grade malignant potential and can extend into an unusual and unexpected site: the thoracic cavity.
Figures and Tables
Fig. 1
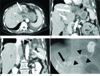
The 82-year-old female with biliary intraductal mucinous papilloma with direct extension into the left thoracic cavity.
A. Axial CT image shows markedly disproportionally dilatated left intrahepatic duct (arrow) without intramural nodule.
B. Coronal CT image reveals the markedly disproportionally dilatated left intrahepatic duct (arrow) without intramural nodule.
C. Coronal CT image of the posterior of (B) shows extension to the diaphragm of the dilated bile duct (arrow), the resultant of communication with the left thoracic space leading to empyema (arrowhead).
D. Percutaneous transhepatic cholangiography shows disproportionally dilated left intrahepatic duct (arrow) and communication with the left thoracic space (arrowheads).
References
1. Kloek JJ, van der Gaag NA, Erdogan D, Rauws EA, Busch OR, Gouma DJ, et al. A comparative study of intraductal papillary neoplasia of the biliary tract and pancreas. Hum Pathol. 2011; 42:824–832.
2. Lim JH, Jang KT, Choi D. Biliary intraductal papillary-mucinous neoplasm manifesting only as dilatation of the hepatic lobar or segmental bile ducts: imaging features in six patients. AJR Am J Roentgenol. 2008; 191:778–782.
3. Lim JH, Yoon KH, Kim SH, Kim HY, Lim HK, Song SY, et al. Intraductal papillary mucinous tumor of the bile ducts. Radiographics. 2004; 24:53–66. discussion 66-67.
4. Kim HJ, Kim MH, Lee SK, Yoo KS, Park ET, Lim BC, et al. Mucin-hypersecreting bile duct tumor characterized by a striking homology with an intraductal papillary mucinous tumor (IPMT) of the pancreas. Endoscopy. 2000; 32:389–393.
5. Yeh TS, Tseng JH, Chiu CT, Liu NJ, Chen TC, Jan YY, et al. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann Surg. 2006; 244:248–253.
6. Sugiyama M, Atomi Y. Intraductal papillary mucinous tumors of the pancreas: imaging studies and treatment strategies. Ann Surg. 1998; 228:685–691.
7. Jang KT, Kwon GY, Ahn G. Clinicopathological analysis of growth patterns of malignant intraductal papillary mucinous tumors of the pancreas: unusual growth pattern of fistulous extension. Korean J Pathol. 2007; 41:38–43.
8. Shibahara H, Tamada S, Goto M, Oda K, Nagino M, Nagasaka T, et al. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004; 28:327–338.
9. Rosai J. Rosai and Ackerman's surgical pathology. 2004. Philadelphia: Elsevier;p. 1078–1080.
10. Kaye MD. Pleuropulmonary complications of pancreatitis. Thorax. 1968; 23:297–306.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download