Abstract
Transcatheter arterial chemoembolization (TACE) is known to be an effective palliative treatment for unresectable hepatocellular carcinoma (HCC). Serious complications, such as neutropenic sepsis and hepatic decompensation, are well known. A HCC rupture following TACE is a rare complication; however, it can be life-threatening if it occurs. In a 75-year-old male patient who subsequently developed capsular rupture of the lipiodol laden mass and several free intraperitoneal chemoembolization agents with hemoperitoneum, we report a case of a ruptured HCC that superficially located arterial enhancing and early wash-out mass in the right hepatic dome following TACE.
Although operative resection is the preferred treatment method for hepatocellular carcinoma (HCC), 70-80% of patients are diagnosed at the intermediate to advanced stages and, therefore, resection is not suitable. Transcatheter arterial chemoembolization (TACE) is the most widely used primary treatment in patients with HCC who are considered to be unsuitable candidates for surgery (1). Even though a survival benefit can be achieved with TACE, it is associated with adverse events that cause significant morbidity and mortality. Fever, ileus, neutropenic sepsis, and hepatic decompensation are common complications; however, ruptured HCC following TACE has rarely been reported (2).
We describe a case of a fatal complication in a patient with a single, superficial HCC who underwent TACE. He had a significant intraperitoneal bleed secondary to tumor rupture immediately following the procedure.
A 75-year-old male with a history of chronic hepatitis B, hypertension, and insulin dependent diabetes was incidentally diagnosed with HCC. Computed tomography (CT) revealed a 4 × 4 cm well circumscribed hypervascular mass in segment VIII (Fig. 1A, B). His liver enzymes and liver function tests were normal, except for a mild elevation of serum alpha-fetoprotein value [11.7 ng/mL (normal < 7.0 ng/mL)]. He underwent TACE, although he had Child Pugh class A (6 points), because of his old age, which makes operation unbearable.
Access for TACE was performed under sterile conditions, with the patient under local anesthesia, by way of the right common femoral artery using a 5 Fr sheath (Radifocus Introducer II; Terumo, Tokyo, Japan) in a retrograde fashion. After imaging the abdominal aorta, superior mesenteric artery angiography was performed using a 5 Fr catheter (Radifocus Introducer II; Yashiro, Tokyo, Japan). On indirect portography, signs of portal venous thrombosis did not appear. With the same catheter, the celiac trunk angiography was undertaken, and the catheter was placed in the right hepatic artery using an angled hydrophilic 0.035 inch Radifocus guidewire (Radifocus Introducer II; Terumo, Tokyo, Japan). Angiography with high-pressure contrast injection showed many small feeding vessels of the capacious tumor burden in liver segment VIII (Fig. 1C). The presence of arteriovenous shunts or extrahepatic collateral vessels could be excluded.
The tumor was embolized with a combination of 40 mg of Adriamycin and 10 mL of Lipiodol after selective catheterization of the appropriate hepatic artery. After embolization, Fentanyl (100 ug) was administered intravenously for pain relief. Before and after embolization, sufficient IV hydration was provided. The procedure was terminated at the point of stop-flow within the main feeding vessels (Fig. 1D). There were no major intraprocedural complications.
Six hours after the procedure, however, the patient became hypotensive (systolic/diastolic blood pressure = 85/47 mm Hg), tachycardic (heart rate = 110 beats per minute) and developed dizziness, nausea, sweating, and moderate abdominal pain. His hemoglobin level had decreased from 12.9 to 10.7 g/dL.
A CT scan revealed a large hemoperitoneum with no active arterial contrast extravasation from the tumor (Fig. 1E). Scattered punctate foci of hyperattenuated particles located in the right paracolic gutter and pelvic cavity suggested free intraperitoneal chemoembolization agents (Fig. 1F). In spite of the ruptured HCC, the patient was controlled by conservative management. He required 6 units of packed red blood cells and 12 units of fresh frozen plasma. Finally, he recovered from the intraperitoneal hemorrhage. He is alive after a 6-month follow-up without tumor recurrence.
TACE of the liver is recommended by the Society of Interventional Radiology as a first-line treatment for inoperable HCC in patients who exceed the Milan or University of California San Francisco criteria but still have a well-preserved liver function (3). TACE combines the effect of targeted chemotherapy with the effect of ischemic necrosis induced by arterial embolization.
Even though satisfactory survival results can be achieved with TACE, it can also be associated with adverse events. It is well known that the common complications related to TACE are postembolization syndrome (fever, abdominal pain, nausea, and vomiting), impaired liver function, and leukocytopenia. There are rare and severe complications including neutropenic sepsis, liver failure, liver abscess, tumor rupture, upper gastrointestinal bleeding, bile duct complications, acalculous cholecystitis, pulmonary embolism, spasm or occlusion of the hepatic artery, and acute renal failure, which are associated with significant morbidity and mortality (4). Previous data indicates a 4.1% treatment-related mortality rate associated with TACE (5). However, spontaneous rupture related to TACE has rarely been reported, and it is associated with an overall mortality rate of 50% along with poor long-term survival rates (6). The incidence is very low, ranging from 0.15% to 0.68%, according to the article (2).
According to clinical literature, large HCC lesions are considered to be at an increased risk of rupture, as they are more likely to contact the liver surface, and also have extrahepatic protrusion (2). Battula et al. (6) reported two cases of ruptured HCC following TACE. These tumors had a large tumor size, male sex, and an exophytic growth of tumor. In our patient, the tumor was ruptured following TACE though a relatively small size. However, the tumor had a superficial location and an exophytic growth, similar to the previous clinical literature.
The pathophysiologic mechanisms of HCC rupture after TACE are not yet fully known. It can be thought to be related to increased pressure inside the friable tumor after TACE, as a result of rapid edematic expansion due to tumor necrosis. This change is expected to be common in a large tumor undergoing the first session of treatment. The tumor may then rupture with or without minor trauma including diaphragmatic movement on the right lobe of the liver. The mechanism of rupture is also thought to be related to vascular injury secondary to embolization. The arterial wall becomes fragile because of inflammatory and reparative changes (78). However, further studies are required to delineate the predisposing factors and mechanisms of rupture of HCC after TACE.
Patients with ruptured HCC present right upper abdominal pain and abdominal distension. The diagnosis is usually confirmed by ultrasonography or CT scan. CT imaging is the most useful modality in the imaging of HCC and can detect most of the complications following TACE. In a ruptured HCC, bleeding or gas (as a result of necrosis) may be seen in the lesion, around the liver or within the peritoneal cavity (9).
Sun et al. (2) reported five cases of ruptured HCC which developed within 16 hours to 7 months after 1005 TACEs. These patients received a repeat emergency arterial embolization for immediate hemostasis and tumor stabilization. In our experience, HCC rupture occurred 6 hours after TACE and then immediate hemostasis was achieved by conservative management. Conservative therapy has been justified for selected patients in hemodynamically stable conditions or in extremely poor conditions; however, a high mortality rate due to continuous bleeding or rebleeding after conservative treatment has been reported (6). The primary objective in the management of these patients is to achieve hemostasis by surgical, non-surgical, or conservative methods. The mortality and morbidity rate is high because the patients usually have a poor reserve and an advanced disease. In our opinion, emergency embolization is an efficient hemostatic treatment in the case of intraperitoneal bleeding of a ruptured HCC. In this particular situation of our patient with bad general health and the absence of active bleeding, embolization had to be excluded. The only solution that was left was conservative management.
In conclusion, TACE is generally a safe procedure, yet, tumor rupture remains a potential complication. In our experience, the superficial location with extracapsular extension of the tumor is a more important risk factor for tumor rupture following TACE than the tumor size. This complication may appear immediately after the procedure, thus, close observation and appropriate management are mandatory.
Figures and Tables
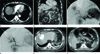 | Fig. 1A 75-year-old male who was incidentally diagnosed with HCC.
A. Arterial phase axial contrast enhanced CT scan of the upper abdomen shows a superficially located, enhanced tumor (arrow) in segment VIII before hepatic chemoembolization.
B. Venous phase coronal CT scan reveals early wash-out of the mass (arrow).
C. Angiography of the celiac trunk before chemoembolization indicated a hypervascular hepatic mass (arrow) with numerous feeding vessels in the right hepatic dome.
D. Hepatic angiography after chemoembolization revealed complete devascularization in the lesion (arrow).
E, F. Axial contrast enhanced CT scan obtained one day after TACE shows a capsular rupture at the level of the lipiodol laden lesion (arrow) and several free intraperitoneal chemoembolization agents with hemoperitoneum (open arrows).
Note.-HCC = hepatocellular carcinoma, TACE = transcatheter arterial chemoembolization
|
References
1. Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002; 35:519–524.
2. Sun JH, Wang LG, Bao HW, Lou JL, Cai LX, Wu C, et al. Emergency embolization in the treatment of ruptured hepatocellular carcinoma following transcatheter arterial chemoembolization. Hepatogastroenterology. 2010; 57:616–619.
3. Brown DB, Geschwind JF, Soulen MC, Millward SF, Sacks D. Society of Interventional Radiology position statement on chemoembolization of hepatic malignancies. J Vasc Interv Radiol. 2009; 20:7 Suppl. S317–S323.
4. Xia J, Ren Z, Ye S, Sharma D, Lin Z, Gan Y, et al. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J Radiol. 2006; 59:407–412.
5. Berger DH, Carrasco CH, Hohn DC, Curley SA. Hepatic artery chemoembolization or embolization for primary and metastatic liver tumors: post-treatment management and complications. J Surg Oncol. 1995; 60:116–121.
6. Battula N, Srinivasan P, Madanur M, Chava SP, Priest O, Rela M, et al. Ruptured hepatocellular carcinoma following chemoembolization: a western experience. Hepatobiliary Pancreat Dis Int. 2007; 6:49–51.
7. Ritter CO, Wartenberg M, Mottok A, Steger U, Goltz JP, Hahn D, et al. Spontaneous liver rupture after treatment with drug-eluting beads. Cardiovasc Intervent Radiol. 2012; 35:198–202.
8. Liu CL, Ngan H, Lo CM, Fan ST. Ruptured hepatocellular carcinoma as a complication of transarterial oily chemoembolization. Br J Surg. 1998; 85:512–514.
9. Bruls S, Joskin J, Chauveau R, Delwaide J, Meunier P. Ruptured hepatocellular carcinoma following transcatheter arterial chemoembolization. JBR-BTR. 2011; 94:68–70.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download