Abstract
A 32-year-old woman had been referred to our hospital for lower abdominal pain. Pelvic ultrasonography and magnetic resonance imaging revealed a huge solid mass with an internal cystic portion. The patient underwent a staging laparotomy and subsequent total abdominal hysterectomy with bilateral salpingo-oophorectomy, bilateral pelvic lymph nodes sampling, and total omentectomy. At staging laparotomy, a large omental mass was found. The tumor displayed the typical histological patterns observed in the yolk sac tumor. The alpha-fetoprotein (AFP) serum value on the 10th day after surgery was 11,576.67 IU/mL and decreased to 6.46 IU/mL after chemotherapy. At the end of the treatment, all the findings, including the AFP level, were normal. We report a case of primary yolk sac tumor of the omentum in a 32-year-old woman.
A yolk sac tumor is the second most common malignant ovarian germ cell tumor, however a yolk sac tumor arising in the omentum is an exceedingly rare malignancy. Yolk sac tumors, also known as endodermal sinus tumors usually occur in the gonads, such as the testis or ovary. But, about 20% arise in extragonadal sites, including the mediastinum, sacrococcygeal region, cervix, vulva, pelvis, and retroperitoneum (12). To our knowledge, a yolk sac tumor has been reported in some case reports. We report a case of primary yolk sac tumor of the omentum in a 32-year-old female and discuss the imaging finding with a review of the literature.
A 32-year-old married woman was referred to our hospital because of lower abdominal pain and distension for 1 month. She had no gynecologic history. She underwent a diagnostic laparoscopy due to infertility 1 year ago. Her menstrual cycle was regular at 28-day intervals with normal flow. She denied any family medical history of malignant disease. On pelvic examination, a huge solid mass was palpated in the posterior side of uterus. The patient's abdomen bulged, making it appear similar to a full-term pregnancy. Pelvic ultrasonography showed 8 × 16 × 18 cm heterogeneous hyperechoic solid mass with an internal anechoic portion. Color Doppler ultrasound showed internal hypervascularity of the tumor (Fig. 1). A pelvic and abdominal magnetic resonance imaging (MRI) revealed a multi-lobulated well enhancing solid and cystic pelvic mass with some hemorrhagic portion adhered to the omentum and some ascites. There was no visible normal ovarian-like structure (Fig. 2). Positron emission tomography showed increased fludeoxyglucose uptake in the solid portion of the tumor, which is suggestive of a malignant tumor (Fig. 3). There was no significantly enlarged lymph node in both the iliac chains and retroperitoneal space. Our preoperative diagnosis was primary ovarian cancer with carcinomatosis. Chest radiography, esophagogastroduodenoscopy, and colonoscopy were negative for additional masses. Serum cancer antigen 125 (CA 125) was 364 U/mL (normal level: less than 35 U/mL). And, other tumor markers including carcinoembryonic antigen and carbohydrate antigen 19-9 (CA 19-9) were within the normal range.
The patient underwent staging laparotomy, which revealed a 20 × 14 cm sized multi-lobulated solid mass in the greater omentum with 1 L of ascites. The result of this frozen biopsy taken from the omental mass was a poorly differentiated either primary or metastatic tumor. Subsequently, a total abdominal hysterectomy with bilateral salpingo-oophorectomy, bilateral pelvic lymph nodes sampling, total omentectomy, and incidental appendectomy were carried out with no macroscopic residual disease.
Histological evaluation of the specimen exhibited the Schiller- Duval body. Careful examination of the ovaries showed no neoplastic involvement. Special immunohistochemical staining of the tumor was strongly positive for alpha-fetoprotein (AFP) and cytokeratin, but was negative for human chorionic gonadotropin and vimentin (Fig. 4).
Although we did not evaluate the preoperative baseline serum AFP, AFP levels on the 10th days after surgery was 11,576.67 IU/mL (normal level: less than 7 IU/mL). At the 2 weeks after surgery, the patient underwent combined intravenous chemotherapy (BEP regimen) with bleomycin 20 units/m2, cisplatin 20 mg/m2, and etoposide 100 mg/m2 for 3 days every 3 weeks over six cycles. After the 4 months of surgery, AFP was 6.46 IU/mL. The patient was followed up for 1 year without clinical and radiologic evidence of recurrence. And, tumor markers were within the normal range.
Yolk sac tumor is a highly malignant neoplasm of germ cells that grows rapidly and metastasizes early via the lymphatic and hematogenous routes (3). Although it usually occurs in the gonads, it has been reported to arise primarily in structures within and outside of the genital tract (24). The sacrococcygeal region and anterior mediastinum are the most common extragonadal locations and the abdomen, and the retroperitoneum are relatively uncommon sites of yolk sac tumor (12).
The omentum is composed mainly of fat, but it contains various tissues including vessels, lymphatics, as well as the immune system and the reported primary tumors of the omentum, although each of them remains very rare and include leiomyosarcomas, fibrosarcomas, liposarcomas, desmoids tumors, mesotheliomas, and gastrointestinal stromal tumors (5).
Three main hypotheses have been proposed to explain the existence of germ cell tumors in extragonadal locations (167). One hypothesis is that it is the origin from an aberrant differentiation of somatic cells. A second hypothesis is that the tumor originates from germ cells that have been misplaced or arrested in their embryonic migration. The third possible mechanism is metastasis from an occult focus in the ovary. In our case, a histopathologic examination of the ovaries excluded this hypothesis.
The histologic pattern of yolk sac tumor is rather typical and is characterized by the presence of embryonic structures resembling the normal fetal yolk sac, Schiller-Duval bodies (a glomerular-like structure with a central vessel surrounded by prominent large cuboidal cells: the presence of this structure is only 20% of yolk sac tumor cases), hyaline periodic acid Schiff-positive intracellular, and extracellular globules (1). In our case, Schiller Duval bodies were seen, and special immunohistochemical staining for AFP and cytokeratin was positive.
A number of radiologic studies have been conducted on yolk sac tumor of ovary, and these found that yolk sac tumors were smooth marginated, well-enhancing tumors that consist of mixed solid and cystic tissue with some areas of hemorrhage and necrosis on both CT and MRI (89). Moreover, the tumor showed a bright dot sign, presumably produced by a well enhanced dilated vessel in the post contrast CT image and prominent signal voids on MRI (9). About 20% of yolk sac tumors arise in extragonadal sites, including the mediastinum, sacrococcygeal region, cervix, vulva, pelvis, and retroperitoneum (1). Although rare, imaging findings of omental yolk sac tumors are similar to yolk sac tumors of the ovary. These tumors show a multi-lobulated solid pelvic mass and small cystic portion with slightly heterogeneous low density on CT (110). The treatment of choice is surgery combined with postoperative chemotherapy (10).
In conclusion, omental yolk sac tumors were visualized as a multi-lobulated solid mass with some hemorrhage and cystic or necrotic portion, as well as a striking enhancement and numerous signal voids, both of which indicate their hypervascular nature. These imaging findings seem to have an important implication for the diagnosis of this tumor.
Figures and Tables
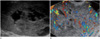 | Fig. 1Ultrasonography of the omental yolk sac tumor in a 32-year-old woman.
A. Pelvic ultrasonography reveals a huge heterogeneous echogenic solid mass with internal anechoic portion.
B. Color Doppler ultrasound shows internal hypervascularity of the tumor.
|
 | Fig. 2Magnetic resonance imaging (MRI) of the omental yolk sac tumor in a 32-year-old woman.
A. Axial T1-weighted MR image shows a multi-lobulated solid and cystic pelvic mass with some areas of hemorrhage (asterisk).
B. Axial T2-weighted MR image shows multi-lobulated solid and cystic mass with internal signal voids (arrowheads) within the tumor due to a rich vascular supply.
C. Axial postcontrast MR image shows a striking enhancement of the mass except for the area of hemorrhage and cysts.
|
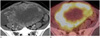 | Fig. 3Positron emission tomography computed tomography (PET-CT) of the omental yolk sac tumor in a 32-year-old woman.
A. Post-contrast CT scan shows an enhancing multi-lobulated solid mass with an internal cystic and necrotic portion.
B. PET shows intense FDG uptake in the solid portion of the tumor, which suggests a malignant tumor.
Note.-FDG = fludeoxyglucose
|
References
1. Kim SW, Park JH, Lim MC, Park JY, Yoo CW, Park SY. Primary yolk sac tumor of the omentum: a case report and review of the literature. Arch Gynecol Obstet. 2009; 279:189–192.
2. Clement PB, Young RH, Scully RE. Extraovarian pelvic yolk sac tumors. Cancer. 1988; 62:620–626.
3. Gooneratne S, Keh P, Sreekanth S, Recant W, Talerman A. Anterior mediastinal endodermal sinus (yolk sac) tumor in a female infant. Cancer. 1985; 56:1430–1433.
4. Dede M, Pabuccu R, Yagci G, Yenen MC, Goktolga U, Gunhan O. Extragonadal yolk sac tumor in pelvic localization. A case report and literature review. Gynecol Oncol. 2004; 92:989–991.
5. Sompayrac SW, Mindelzun RE, Silverman PM, Sze R. The greater omentum. AJR Am J Roentgenol. 1997; 168:683–687.
6. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649–655.
7. Jones MA, Clement PB, Young RH. Primary yolk sac tumors of the mesentery. A report of two cases. Am J Clin Pathol. 1994; 101:42–47.
8. Yamaoka T, Togashi K, Koyama T, Ueda H, Nakai A, Fujii S, et al. Yolk sac tumor of the ovary: radiologic-pathologic correlation in four cases. J Comput Assist Tomogr. 2000; 24:605–609.
9. Choi HJ, Moon MH, Kim SH, Cho JY, Jung DC, Hong SR. Yolk sac tumor of the ovary: CT findings. Abdom Imaging. 2008; 33:736–739.
10. Xinghui Y, Jing H, Mingju L, Weizhong G. Endodermal sinus tumour of the omentum in a child. Pediatr Radiol. 2004; 34:985–987.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download