Abstract
We present a rare case of a myxoid matrix-rich mesenteric lipoblastoma. In this case, fat-saturated T1-weighted magnetic resonance (MR) imaging failed to disclose the fatty component of the tumor, while in-phase and opposed-phase MR imaging could accurately identify small fat scattered in this myxoid matrix-rich tumor.
Lipoblastoma is a rare benign tumor composed of embryonic fat that most often presents in children younger than 3 years of age (1234). Imaging findings of the tumor reflect the histopathologic features typically showing a mixture of mature and immature adipocytes, often with an abundant myxoid matrix (123). In most cases, the imaging diagnosis of lipoblastoma can be made in young children without difficulty by identifying fat in the tumor on CT or magnetic resonance (MR) imaging (12). The presence of this fat component of lipoblastoma is generally confirmed by T1-weighted MR imaging without and with fat saturation, which usually obviates the additional use of T1-weighted gradient-echo in-phase and opposed-phase MR imaging in identifying fat in fatty tumors (5). Herein, we present a case with myxoid matrix-rich mesenteric lipoblastoma in whom the presence of fat in the tumor was identified by a diffuse signal drop on opposed-phase MR imaging, providing a valuable diagnostic clue.
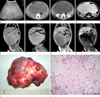
A 13 month-old boy had progressive abdominal distension for 2 months. A large, soft and mobile abdominal mass was detected during a physical examination. All laboratory results were unremarkable. Abdominal sonography showed a large, lobulated, solid, intra-peritoneal mass with homogeneously increased echogenicity (Fig. 1A). Pre- and post-contrast abdominal CT using a 4-detector row CT system (Lightspeed QX/i; GE Healthcare, Milwaukee, WI, USA) was performed with 100 kV and 120 mAs. The intra-peritoneal mass consisted of multiple lobules showing central isodensity (36 HU on pre-contrast scan, 40 HU on post-contrast scan) and peripheral hypodensity (10 HU on pre-contrast scan, 17 HU on post-contrast scan), without evidence of fat density on pre-contrast abdominal CT (Fig. 1B). Post-contrast CT showed no discernible contrast enhancement in the mass (Fig. 1C). Abdominal MR imaging (Gyroscan Intera; Philips Healthcare, Best, The Netherlands) was requested for further characterization of the mass. A pediatric radiologist who attended the MR examination added in-phase and opposed phase imaging to a routine protocol for abdominal mass evaluation. The mass demonstrated diffuse T2 hyperintensity with hypointense septum-like structures (Fig. 1D) and T1 signal intensity, which was slightly hyperintense to the muscle with hyperintense rims (Fig. 1E). Fat-saturated spin-echo T1 sequence (TR/TE 635.5/15.0 ms, flip angle 90°), using spectral presaturation with inversion recovery, failed to show unequivocal evidence of fat in the mass (Fig. 1F). T1-weighted gradient-echo in-phase (TR/TE 131.2/4.6 ms, flip angle 80°) and opposed-phase (TR/TE 166.0/2.4 ms, flip angle 80°) sequences were subsequently used to identify the presence of fat in the mass. Opposed-phase images showed a marked signal drop of the entire mass (Fig. 1G, H), indicating the presence of numerous fat-water interfaces in the mass.
At surgery, a huge (17 × 12 × 8 cm), lobulated, myxomatous solid mass arising from the mesentery was identified along with a segment of the small intestine sturdily adhered to the mass. The mass was completely resected along with the adhered segment of the small bowel. Grossly, the mass appeared bosselated, yellowish gray, and myxoid (Fig. 1I). Microscopically, the encapsulated tumor was composed abundant of myxoid stroma, scattered immature lipoblasts, a few mature adipocytes, and multiple fibrovascular septa, which was consistent with the characteristics of lipoblastoma (Fig. 1J).
Lipoblastoma is an uncommon benign mesenchymal tumor that occurs almost exclusively in infants and young children (1234). The tumor more commonly occurs in boys (123). Common locations of the tumor include the soft tissues of the extremities or the trunk (1234). Abdominal lipoblastoma accounts for approximately 7% of all lipoblastomas and is rarely located in the mesentery (4). The most common presentation of lipoblastoma is a steadily growing, soft, and non-tender mass. Symptoms are usually related to the location and size of the tumor.
Tumor cells of lipoblastoma demonstrate immunohistochemical and ultrastructural similarities to yellow fat, suggesting that the tumor may be a consequence of continued proliferation of immature fat cells in the postnatal period (4). In fact, lipoblastoma is histologically composed of monovacuolated and multivacuolated lipoblasts, mesenchymal cells, a plexiform capillary network, myxoid stroma, and mature adipocytes that are organized in lobules separated by fibrous septa. The myxoid component may be prominent in very young patients (2).
The imaging findings of lipoblastoma may vary depending on its composition; especially according to the proportion of myxoid tumor matrix of the tumor (23). The typical but nonspecific sonographic appearance of the tumor is a homogeneous hyperechoic solid mass, while the tumor may infrequently show mixed echogenicity and fluid-filled spaces (23). CT and MR imaging often provide a diagnostic clue to lipoblastoma, as well as other fatty tumor such as lipoma or liposarcoma, by demonstrating a predominantly fatty mass (12). In our case, an abdomen sonography showed a homogeneously echogenic mass. The CT findings of the non-enhancing hypodense mass without any measurable fat were also non-specific, as in the sonographic findings, which suggests lipoblastoma. In usual lipoblastoma, measurable fat on CT is present in the tumor. In MR examination, the tumor appeared hyperintense with multiple hypointense septa on T2-weighted images, as described in the literature (23). Lipoblastoma usually shows hyperintensity on T1-weighted images and substantial signal drop on fat-saturated T1-weighted images (123). However, the tumor of our case appeared unusually isointense without definite evidence of fat on T1-weighted images. Fat-saturated T1-weighted MRI could not identify fat in the mass. These unusual CT and MRI findings might be attributed to abundant myxoid matrix with finely scattered small fat lobules in the tumor on the histologic examination. In addition, immature lipoblasts tend to show signal intensity lower than that of mature lipocytes on T1-weighted images (3).
Signal drop on opposed-phase MR images has been used as an important finding in revealing diffuse fatty infiltration in the liver and the thymus, as well as minimal fat in fatty tumors (5678). In principle, the signal loss on opposed-phase MR images occurs only for voxels containing both fat and water (78). The signal drop is maximized when the signal intensity of water is almost equal to that of fat. Opposed-phase imaging is recommended for demonstrating lesions with small amounts of fat, while fat suppression on frequency-selective fat-saturated imaging is ineffective when the fraction of adipose tissue is water (7). In our case, this may explain the discrepancy between the two fat-suppressed techniques in demonstrating a small amount of intra-tumoral fat. Hence, the signal loss of the tumor on opposed-phase MR images, in our case, was not conspicuous on frequency-selective fat-saturated MR images, and provided an important diagnostic clue to a fat-containing tumor. The unusual hyperintensity on T2-weighted imaging and hypointensity isointense to the muscle on T1-weighted imaging were described in a case of lipoblastoma with lipoblasts and mature lipocytes in a myxoid matrix (9). However, the use of opposed-phase MR imaging for demonstrating an unusually small amount of fat in lipoblastoma has, to the best of our knowledge, not been reported.
The differential diagnoses of a fat-containing mass include lipoma, liposarcoma, and teratoma (23). Lipoma can be distinguished from other fat-containing tumors by the absence of non-fatty mesenchymal tissue in the tumor and old age (3). Teratoma is commonly seen in young children and can easily be diagnosed by recognizing fat, calcification, and the presence of a cyst in the tumor. However, we should know that it may be difficult to distinguish lipoblastoma from teratoma without calcification (2). On the other hand, the imaging and histologic findings of liposarcoma may simulate those of lipoblastoma, and liposarcoma is rare in young children (1234).
Complete surgical resection is recommended for the treatment of lipoblastoma and is usually curative. Local recurrence occurs in less than 25% of patients (1234). Since the local recurrence may spontaneously mature or regress, mutilating surgery for complete resection is not mandatory (24).
We present a case with myxoid matrix-rich mesenteric li-poblastoma in whom the presence of fat in the tumor was identified by a diffuse signal drop on opposed-phase MR imaging, providing a valuable diagnostic clue.
Figures and Tables
Fig. 1
Imaging and histologic findings of myxoid matrix-rich mesenteric lipoblastoma.
A. Transverse abdominal US shows a large, lobulated, well-defined, hyperechoic solid mass without posterior acoustic enhancement.
B. Precontrast axial CT image reveals multiple lobules of the mass with central isodensity (36 HU) and peripheral hypodensity (10 HU) and without a definite evidence of fat.
C. Contrast-enhanced axial CT image demonstrates a minimal degree of enhancement (4-7 HU) in the hypodense mass.
D. Axial T2-weighted MR image shows diffuse hyperintensity with hypointense septa of the mass.
E. Coronal T1-weighted MR image shows diffuse isointensity with hyperintense peripheral rims of the mass.
F. Coronal, fat suppressed T1-weighted MR image shows no evidence of fat suppression in the majority of the mass.
G. Coronal, T1-weighted gradient-echo in-phase MR image shows a slightly hypointense mass with hyperintense peripheral rims.
H. Coronal opposed-phase MR image demonstrates a considerable drop in signal intensity in the entire mass.
I. Gross specimen shows that the resected mass appears bosselated, yellowish gray, and myxoid.
J. Photomicrograph shows scattered immature lipoblasts (long arrow) characterized by nuclear indentation by lipid-containing cytoplasmic vacuoles, as well as a few mature adipocytes (short arrow) characterized by the crescent-shaped peripheral nucleus and a large lipid droplet in abundant myxoid matrix background (Hematoxylin & Eosin stain, × 40).
Note.-MR = magnetic resonance
References
1. Bancroft LW, Kransdorf MJ, Peterson JJ, O'Connor MI. Benign fatty tumors: classification, clinical course, imaging appearance, and treatment. Skeletal Radiol. 2006; 35:719–733.
2. Moholkar S, Sebire NJ, Roebuck DJ. Radiological-pathological correlation in lipoblastoma and lipoblastomatosis. Pediatr Radiol. 2006; 36:851–856.
3. Mo YH, Peng SS, Li YW, Shun CT. Mesenteric lipoblastoma: case report. Pediatr Radiol. 2003; 33:37–40.
4. Cudnik R, Efron PA, Chen MK, Reith JD, Beierle EA. Mesenteric lipoblastoma: a rare location in children. J Pediatr Surg. 2008; 43:e5–e7.
5. Kelekis NL, Alexopoulou E, Brountzos EN, Ladis V, Boussiotou A, Kelekis DA. Giant adrenal myelolipoma with minimal fat content in a patient with homozygous beta-thalassemia: appearance on MRI. J Magn Reson Imaging. 2003; 18:608–611.
6. Takahashi K, Inaoka T, Murakami N, Hirota H, Iwata K, Nagasawa K, et al. Characterization of the normal and hyperplastic thymus on chemical-shift MR imaging. AJR Am J Roentgenol. 2003; 180:1265–1269.
7. Delfaut EM, Beltran J, Johnson G, Rousseau J, Marchandise X, Cotten A. Fat suppression in MR imaging: techniques and pitfalls. Radiographics. 1999; 19:373–382.
8. Merkle EM, Nelson RC. Dual gradient-echo in-phase and opposed-phase hepatic MR imaging: a useful tool for evaluating more than fatty infiltration or fatty sparing. Radiographics. 2006; 26:1409–1418.
9. Wang SF, Chang CY, Wu HD. Lipoblastomatosis of the shoulder: unusual MR appearance. Br J Radiol. 1998; 71:884–885.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download