Abstract
Purpose
To evaluate CT findings and radiologic outcomes of pulmonary lipiodol accumulation (PLA) after transarterial chemoembolization (TACE).
Materials and Methods
This retrospective study involved 488 TACEs for hepatocellular carcinoma (HCC) (n = 160) and hepatic metastasis for non-hepatic malignancies (n = 7) in 167 patients. We reviewed the patient clinicoradiologic findings before and after TACE and calculated the incidence of PLA and PLA resolution time after initial CT and after TACE.
Results
Lipiodol accumulation in the lungs was seen under CT after TACE in seven patients (M : F = 6 : 1, mean age 61 years). The incidence of PLA at CT was 4.1% (7/167 patients). In five patients, associated intrathoracic abnormalities including pleural effusion with (n = 3) or without consolidation (n = 2) were revealed at CT scans. The CT resolution time and PLA recovery time were 56 ± 54 days and 66 ± 52 days, respectively.
Transarterial chemoembolization (TACE) is widely used for the treatment of hepatocellular carcinoma (HCC) by the obstruction of hepatic arterial flow using gelfoam, iodized oil (lipiodol), and cytotoxic agents (adriamycin) (12). TACE is known to be a safe technique for selectively targeting tumors; however, several complications in non-target organs have been reported including post-embolization syndrome (fever, abdominal pain, nausea, vomiting), ischemic cholecystitis, splenic infarction, gastrointestinal mucosal lesions, pulmonary lipiodol embolism (PLE) and infarction, spinal cord injury, and ischemic skin lesions (12). PLE with or without pulmonary lipiodol accumulation (PLA) is a rare pulmonary complication after TACE, which can occur by the accidental perfusion of lipiodol into the arteriovenous shunts and inferior phrenic artery that subsequently result in the accumulation of lipiodol in the pleura and lung (12).
Radiographic findings of PLE reported by Chung et al. (3) identified diffuse bilateral pulmonary infiltrate and pleural effusion. In a CT study of pulmonary complications after TACE, Tajima et al. (4) reported that CT findings of pulmonary complication after TACE included lipiodol accumulation in the lung or pleura, as well as consolidation and pleural effusion. Recently, in a case report of a lipiodol embolism, Choi et al. (5) reported that a lipiodol cast in the pulmonary arteries corresponded to high-attenuation linear branching opacities on non-contrast CT. To our knowledge, there are no reports in the literature about radiologic outcomes of PLA after TACE.
The purpose of our study was to evaluate these CT findings and radiologic outcomes of PLA after TACE.
Between January 2006 and November 2008, one hundred sixty seven patients underwent four hundred eighty eight TACE procedures for HCC (n = 160), cholangiocarcinoma (n = 1), or hepatic metastasis of rectal cancers (n = 3), prostate cancer (n = 1), carcinoma of the ampulla of Vater (n = 1), and urothelial transitional carcinoma (n = 1). We retrospectively reviewed their radiologic and clinical findings before and after TACE. Since this was a retrospective study, institutional review board approval was not required, and patient informed consent was waived. Written informed consent was obtained from all patients for TACE and CT scans. We excluded individuals with previously existing pulmonary calcifications (sequelae of tuberculosis or other granulomatous disease), preexisting lipiodol accumulation or isolated intrathoracic abnormalities (consolidation, pleural effusion or pleural thickening without lipiodol accumulation in the lung or pleura), and patients who did not undergo liver or chest CT scans after TACE from the study.
Hepatic arterial superselection was performed with contrast media (Visipaque, 320 mg I/mL, GE Healthcare, Cork, Ireland) using digital subtraction angiography (Axiom Artis, Siemens, Erlangen, Germany; 70 kV, 297 mA) via the femoral artery using the Seldinger method. A lipiodol emulsion was made out of 8.0 mg adriamycin (Adriamycin-RDF injection, Il-dong, Seoul, Korea) with 2.0 mL normal saline, 6.0 mL contrast media, and 6.0 mL lipiodol (Lipiodol Ultra-Fluide, Guerbet, Aulnay-sous-Bois, France). The amount of lipiodol injected was based on the number and the maximum diameter of the tumors, and Child-Pugh score.
After TACE, chest radiography or CT and liver CT scans were performed to evaluate the post-procedural complications or treatment response. Post-TACE chest radiographs were obtained in 445 TACE procedures from 129 patients, and no post-TACE chest radiographs in 43 TACE procedures from 38 patients. The time interval from TACE to post-TACE 578chest radiography was 25.7 ± 39.3 days (range, 0-194 days; median, 1 day; 0-3 days, 265 Post-TACE chest radiography; 4-30 days, 35; 31-60 days, 61; > 60 days, 84). The liver or chest CT scans were performed using multidetector computed tomography (Lightspeed Pro 16, GE Medical Systems, Milwaukee, WI, USA) at 120 kVp with 3.8 or 5.0 mm scanning thicknesses, respectively. The liver CT scans covered 3 cm above the hepatic dome including the entire liver, and the chest CT scans covered the entire lungs from the apex to the base. The CT contrast medium included 120 mL of non-ionic material with a velocity of 2.8 to 3.0 mL/s for liver scans, and 100 mL non-ionic material with a velocity of 2.0 mL/s for chest scans. All liver or chest CT scans contained non-enhanced images. In all 167 patients, 488 liver CT scans were performed before and after TACE, and 28 chest CT scans were performed for other respiratory symptoms such as fever, cough, dyspnea, hemoptysis or chest pain after TACE. The time interval from TACE to initial post-TACE CT was 24.9 ± 12.7 days (range, 0-146 days; median, 22 day), while the time interval between initial and follow-up post-TACE CT was 53.1 ± 55.4 days (range, 0-651 days; median, 31 days).
A thoracic radiologist with 7 years experience, an interventional radiologist with 21 years experience, and a radiology resident reviewed the radiographic, CT, and angiographic findings by consensus. On chest radiographs and CT scans, the presence or absence of lipiodol accumulation at the pleural surface or in the lung, the location of lipiodol accumulation and associated intrathoracic abnormalities such as consolidation and pleural effusion, were evaluated. On non-enhanced CT, we evaluated the presence or absence of high-attenuation linear branching opacities in the lung.
In patients with PLA, TACE angiographic images were evaluated for tumor location, presence or absence of vascular abnormalities including arterioportal (AP) or arteriovenous (AV) shunt and systemic supplies by the inferior phrenic or hepatic arteries, and the amount of injected pure lipiodol or lipiodol emulsion.
We calculated the incidence of PLA, time interval from TACE to post-TACE chest radiography, initial CT interval (time interval from TACE to initial post-TACE CT), time interval from initial post-TACE CT to post-TACE chest radiography, CT resolution time (time from when PLA disappeared in follow up post-TACE CT after initial post-TACE CT), and PLA recovery time (time from when PLA disappeared in follow up post-TACE CT after TACE), and whether any respiratory symptoms such as fever, cough, dyspnea, or hemoptysis were present after TACE.
Table 1 summarizes the TACE and post-TACE CT findings in patients with PLA.
Seven patients (M : F = 6 : 1; age range, 42-76 years; mean age, 61 years) had lipiodol accumulation in the lung at chest (n = 3) or liver (n = 7) CT after TACE, from which the incidence was 4.1% (7 of 167 patients) (Figs. 1, 2). However, there were no high-attenuation linear branching opacities in the lung on non-enhanced CT. The locations of lipiodol accumulation included the right lower lobe (n = 6), right middle lobe (n = 4), and left lower lobe (n = 1). In five patients, associated intrathoracic abnormalities including pleural effusion with (n = 3) or without consolidation (n = 2), were revealed on CT scans. In all patients, lipiodol accumulation was not detected at the chest radiographs. Two patients had no associated intrathoracic abnormalities at their chest radiographs; however, associated intrathoracic abnormalities were found in five patients, including consolidation (n = 1), pleural effusion (n = 3), or both (n = 1) (Fig. 1).
At TACE, tumor locations included the right hepatic dome (n = 4), the segment 3 (n = 2), and whole liver (n = 1). Of the seven patients examined, one had no detailed information on the angiographic findings including vascular abnormalities, superselected artery, and the injected materials because TACE had been performed at another institute. The hepatic artery was superselected in five patients, and the right inferior phrenic artery was superselected in three patients during TACE. The AP shunt was visible in only one patient, while no AV shunt was visible.
In seven patients with PLA, the time interval from TACE to post-TACE chest radiography was 12.6 ± 14.7 days (range, 0-38 days; median 2 days), the initial CT interval after TACE was 15 ± 13 days (range, 0-34 days), and the time interval from initial post-TACE CT to post-TACE chest radiography was 14.3 ± 24.5 days (range, 0-68 days; median 3 days). The CT resolution and recovery time of PLA was not calculated in two patients because follow-up CT scans were not performed. In five patients, the CT resolution time was 56 ± 54 days (range, 23-150 days; median, 29 days) based on the initial post-TACE CT and 21.4 ± 46.7 days (range, 0-105 days; median, 0 day) based on the last follow up post-TACE CT. The PLA recovery time after TACE was 66 ± 52 days (range, 28-153 days; median, 47 days) (Figs. 1, 2).
Pulmonary lipiodol accumulation (PLA) is a rare and potentially fatal complication of TACE (13). The incidence of PLA has been reported to be 1.8%, 4.5% and 23% based on clinical findings and radiography, CT scans, and perfusion scans, respectively (36). In our study, the incidence of PLA was 4.1% (7 of 167 patients) based on CT scans, which is similar to the incidence reported by Wu et al. (6). Of the four patients with PLA in their study, two patients died of acute respiratory failure. No patients had respiratory failure with a fatal course in our study, although three patients had hemoptysis, fever, and dyspnea.
Lipiodol has been used as a carrier vehicle for therapeutic agents since Raoul et al. (7) reported that lipiodol concentrated mainly in the liver and the lungs after hepatic artery injection. Although the mechanism of PLE after TACE is still unclear, the most probable hypothesis for symptomatic pulmonary injury in PLE is pulmonary capillary leakage and edema caused by a high concentration of unbound free fatty acids resulting from the breakdown of oil, and causing microemboli (3). In severe cases, pulmonary fat embolisms can cause atelectasis or pneumonia due to the reduction of pulmonary surfactants (89).
Since PLE has been first reported in 1990 by Samejima et al. (810), many researchers have evaluated the clinical and radiologic features of PLE. Lörcher et al. (11) proposed four stages of intrathoracic distribution of the contrast medium: stage 1, No contrast medium in the pleura and lung; stage 2, contrast medium only in the pleura; stage 3, contrast medium distributed focally in the lung; and stage 4, contrast medium distributed diffusely in the lobe or segment. Chung et al. (3) described the possible relationship of clinical manifestations such as cough, hemoptysis, and dyspnea, while the radiographic findings such as diffuse bilateral pulmonary, infiltrate after TACE with iodized oil infused into the hepatic artery. Tajima et al. (4) modified the previous Lörcher's classification into five grades of pulmonary complications after TACE: grade I, no definite evidence of intrathoracic abnormality; grade II, intrathoracic abnormalities such as consolidation, pleural effusion, or pleural thickening, although there is no intrathoracic lipiodol accumulation; grade III: lipiodol accumulation at the surface of the pleura; grade IV: scattered lipiodol accumulation in the lung field; and grade V, diffuse lipiodol accumulation in the lung field, occupying more than one pulmonary segment. Recently, in a case report of a lipiodol embolism, Choi et al. (5) reported that a lipiodol cast in the pulmonary arteries corresponded to high-attenuation linear branching opacities on noncontrast CT. In our study, we analyzed the CT findings and CT resolution time of PLA according to lipiodol accumulation in the lung or pleura and high attenuated branching linear pulmonary opacities on CT scans.
In a TACE study, Chung et al. (3) reported diffuse and bilateral chest radiographic abnormalities and fine reticulonodular interstitial or flocculent alveolar opacities. These radiographic findings were most severe two to ten days after TACE, and then slowly disappeared until they completely cleared ten to twenty-eight days later. The presence or absence of lipiodol accumulation in chest radiographs was not evaluated in their study. We evaluated the presence or absence of lipiodol accumulation, but did not detect it in chest radiographs. In our study, consolidations were partially recovered, and pleural effusions were completely (n = 2) or partially (n = 1) improved at CT. Lipiodol accumulation at the pleural surface or in the lung disappeared 47 to 153 days after TACE. To our knowledge, the outcome of lipiodol accumulation at the pleural surface or in the lung has not been reported in the literature. We are the first to report the CT recovery time of PLA, which was 66 days.
The risk factors of PLE after TACE are known to include the amount of lipiodol infused, the presence of an arteriovenous shunt, and embolization via the inferior phrenic artery (34). The amount of lipiodol infused is one of the most important risk factors, especially when more than 20 mL of lipiodol is used (368). In addition, Lin and Kuo (8) reported a significant correlation between the amount of lipiodol used and the time until patient recovery. In our study, no more than 20 mL of lipiodol was injected in all patients with PLA, and all recovered without a fatal course.
Superselective inferior phrenic artery (IPA) angiography is necessary to improve the therapeutic effects before TACE because IPA can be a parasitic artery of abdominal malignancies such as hepatocellular carcinomas, which cause hepatic arterial obstructions (1213). However, in a prospective TACE study using IPA, Tajima et al. (4) reported that the estimated rate of PLE was 52% after TACE. In our retrospective study, the IPA was superselected in 3 of 6 patients with PLA. Therefore, to prevent pulmonary complications, embolization for shunts to pulmonary vessels or hepatic veins is required in TACE via the IPA.
Our study has several limitations. First, we could not statistically analyze PLA risk factors because the number of patients with PLA was too small. This was mainly due to the fact that PLA is a rare complication of TACE. Second, a post-TACE chest radiography was not performed in about 10% of all TACE procedures and time intervals between TACE and post-TACE chest radiography were very variable because our study was retrospective. To our knowledge, there were no reports about the direct visualization of lipiodol accumulation at radiograph in patients with PLE (31415). Therefore, we could calculate the incidence of lipiodol accumulation on CT because post-TACE CT scans were performed in all patients. Third, the coverage of lungs in the liver CT was variable and chest CT scans were not performed in all patients. Fourth, the initial CT interval after TACE was variable and we could not obtain CT images immediately after TACE because our study was retrospective. In the future, a more accurate CT resolution time for PLA should be subscribed through prospective studies.
In conclusion, lipiodol accumulation in the lungs was seen only in CT images, although PLE radiologic findings can include lipiodol accumulation with or without consolidation or pleural effusion. Recovery time of lipiodol accumulation was 66 days. We believe that the clinical and radiologic outcome of PLA without respiratory failure is good, and conservative treatment will be sufficient when lipiodol accumulation in the lungs is seen in CT images after TACE.
Figures and Tables
Fig. 1
A 76-year-old man who presented with hemoptysis after TACE for hepatocellular carcinoma via right inferior phrenic (pure lipiodol 1.0 mL and emulsion 11 mL) and right hepatic arteries (pure lipiodol 1.0 mL and emulsion 3.0 mL) (A). Immediate chest radiograph after TACE (B) shows segmental atelectasis in the right lower lung zone (arrow) as well as right pleural effusions and a lipiodol laden mass lesion (arrowheads) in the right upper abdomen. At 3 days after TACE, a non-enhanced initial chest CT scan (C) shows consolidation with lipiodol accumulation in the right lower lobe (arrow) and right pleural effusions (star). At 153 days after TACE, a non-enhanced follow-up liver CT scan (D) shows complete improvement of the lipiodol accumulation and partial improvement of consolidation in the right lower lobe (arrow), as well as right pleural effusion.
Note.-TACE = transarterial chemoembolization
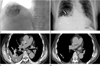
Fig. 2
A 42-year-old man who underwent TACE for hepatocellular carcinoma via both the hepatic and right inferior phrenic arteries (pure lipiodol 3.0 mL and emulsion 11 mL).
At 5 days after TACE, initial non-enhanced chest CT scan (A) shows lobular consolidation with lipiodol accumulation in the posterior basal segment of right lower lobe (arrow) and a lipiodol laden mass lesion in the hepatic dome (star). At 28 days after TACE, a non-enhanced follow-up liver CT scan (B) shows complete improvement of the lobular consolidation with lipiodol accumulation in the posterior basal segment of right lower lobe and no interval change of the lipiodol laden mass lesion in the hepatic dome.
Note.-TACE = transarterial chemoembolization

References
1. Xia J, Ren Z, Ye S, Sharma D, Lin Z, Gan Y, et al. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J Radiol. 2006; 59:407–412.
2. Lee KH, Sung KB, Lee DY, Park SJ, Kim KW, Yu JS. Transcatheter arterial chemoembolization for hepatocellular carcinoma: anatomic and hemodynamic considerations in the hepatic artery and portal vein. Radiographics. 2002; 22:1077–1091.
3. Chung JW, Park JH, Im JG, Han JK, Han MC. Pulmonary oil embolism after transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1993; 187:689–693.
4. Tajima T, Honda H, Kuroiwa T, Yabuuchi H, Okafuji T, Yosimitsu K, et al. Pulmonary complications after hepatic artery chemoembolization or infusion via the inferior phrenic artery for primary liver cancer. J Vasc Interv Radiol. 2002; 13:893–900.
5. Choi SY, Goo JM, Chun HJ, Han DH. Lipiodol can simulate cement embolism in patients having undergone vertebroplasty due to metastasis from hepatocellular carcinoma. AJNR Am J Neuroradiol. 2010; 31:E2–E3.
6. Wu GC, Perng WC, Chen CW, Chian CF, Peng CK, Su WL. Acute respiratory distress syndrome after transcatheter arterial chemoembolization of hepatocellular carcinomas. Am J Med Sci. 2009; 338:357–360.
7. Raoul JL, Bourguet P, Bretagne JF, Duvauferrier R, Coornaert S, Darnault P, et al. Hepatic artery injection of I-131-labeled lipiodol. Part I. Biodistribution study results in patients with hepatocellular carcinoma and liver metastases. Radiology. 1988; 168:541–545.
8. Lin MT, Kuo PH. Pulmonary lipiodol embolism after transcatheter arterial chemoembolization for hepatocellular carcinoma. JRSM Short Rep. 2010; 1:6.
9. Fraimow W, Wallace S, Lewis P, Greening RR, Cathcart RT. Changes in pulmonary function due to lymphangiography. Radiology. 1965; 85:231–241.
10. Samejima M, Tamura S, Kodama T, Yuuki Y, Takasaki J, Sekiva R, et al. [Pulmonary complication following intra-arterial infusion of lipiodol-adriamycin emulsion for hepatocellular carcinoma, report of a case]. Nihon Igaku Hoshasen Gakkai Zasshi. 1990; 50:24–28.
11. Lörcher U, Peters J, Kollath J. [Changes in the lungs and pleura following chemoembolization of liver tumors with mitomycin-lipiodol]. Rofo. 1990; 152:569–573.
12. Charnsangavej C, Chuang VP, Wallace S, Soo CS, Bowers T. Angiographic classification of hepatic arterial collaterals. Radiology. 1982; 144:485–494.
13. Koehler RE, Korobkin M, Lewis F. Arteriographic demonstration of collateral arterial supply to the liver after hepatic artery ligation. Radiology. 1975; 117:49–54.
14. Han D, Lee KS, Franquet T, Müller NL, Kim TS, Kim H, et al. Thrombotic and nonthrombotic pulmonary arterial embolism: spectrum of imaging findings. Radiographics. 2003; 23:1521–1539.
15. Rossi SE, Goodman PC, Franquet T. Nonthrombotic pulmonary emboli. AJR Am J Roentgenol. 2000; 174:1499–1508.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download