Abstract
Carotid stump, the blind remnant of an occluded internal carotid artery, can be a potential source of microemboli, and warrants its exclusion from the vascular lumen to prevent the recurrence of a microembolism. In a 69-year-old male with a symptomatic carotid stump and acute angle between left common carotid artery and aortic arch, a 7-Fr. shuttle sheath was scarcely placed into the left carotid artery but the 7-mm-diameter stent-graft-loading balloon could not be inserted into the 7-Fr. shuttle sheath. With the mounting a stent graft over a 5-mm balloon, the balloon-expandable stent graft was unfolded. The self-expandable stent was placed over the stent graft, and an 8-mm balloon was subsequently expanded. Self-expanding stenting can be useful for troubleshooting in a case of device incompatibility coming from the different calibers of the external and common carotid arteries for the successful exclusion of a symptomatic carotid stump.
The carotid stump is a blind, pouch-like remnant of the occluded internal carotid artery (ICA) at its proximal aspect. When ischemic symptoms persist in a properly compensated ICA occlusion, the carotid stump is a potential source of microemboli (1). The turbulence in the stump contributes to progressive atherosclerosis and thrombogenesis, and a subsequent microembolism may occur through the anastomotic channel between the external carotid artery (ECA) and the distal ICA. Carotid stump syndrome has been reported in 7.5% of carotid endarterectomy series, which were treated by conventional methods, with surgical excision of the stump or oversewing of the ICA origin (2). More recently, either the stump was excluded from the vascular lumen by placing a self-expandable stent graft (Wallgraft endoprosthesis; Boston Scientific, Natick, MA, USA; 345), or acutely near-occluded ICA was reopened anterogradely under proximal balloon protection (67). In our patient, our attempt to place a balloon-expandable stent graft for the exclusion of the stump resulted in some issues; a stent graft mounted on a balloon with a diameter of more than 7 mm is usually compatible with a shuttle sheath of at least 7 Fr., was difficult to advance along the acute angle between the aortic arch and the carotid artery (8). Furthermore, the considerable difference in diameter between the ECA and the common carotid artery (CCA) carries a risk of rupture during balloon expansion (9). We present a bailout technique of sequential self-expandable stenting over the balloon-expandable stent graft for the exclusion of a carotid stump.
A 69-year-old man with a history of hypertension and diabetes mellitus presented with dysarthria and right hemiparesis. Diffusion-weighted magnetic resonance imaging (MRI) revealed multifocal embolic infarctions of the left anterior cerebral artery (ACA) and middle cerebral artery (MCA) territory. Electrocardiography and echocardiography ruled out a cardioembolism. Digital subtraction angiography (DSA) revealed occlusion of both ICAs and the presence of a left ICA stump. The left ophthalmic artery and distal ICA were filled retrogradely from the ECA (Fig. 1). The left ACA was dominant and the right ACA territory was supplied by the left anterior circulation. Three-dimensional rotational angiography revealed that the left ECA and the CCA were 5.5 and 7 mm in diameter, respectively. On the following day, the patient developed left leg weakness (Medical Research Council Scale for Muscle Strength, grade 4), and new diffusion restrictions were found in both the ACA and left MCA territories. One week later, right arm weakness recurred despite systemic heparinization and triple antiplatelet therapy (aspirin, 100 mg; clopidogrel, 75 mg; cilostazol, 200 mg). It was suspected that the stump was the source of the recurrent microembolism. At the initial session, an attempt to place a 9-Fr. shuttle sheath in the left CCA failed, but a 7-Fr. shuttle sheath (Flexor Tuohy-Borst Sidearm Introducers, Cook, Bloomington, IN, USA) was successfully placed. However, a stent-graft-loading balloon with a diameter of 7 mm could not be inserted into the 7-Fr. shuttle sheath. At a second session, a 7-Fr. shuttle sheath was placed into the CCA under 5,000 units of systemic heparinization. An 0.018-inch SV guidewire (Boston Scientific, Natick, MA, USA) was passed into the ECA branch, and a Jostent peripheral stent graft (4-9 mm × 38 mm; Abbott Vascular, Rangendingen, Germany), which was mounted over a Savvy balloon (5 × 30-mm; Cordis, Miami, FL, USA), was placed into the left ECA-CCA. After balloon expansion at 6 Atm, the distal portion of the stent graft was fitted to the ECA, but the proximal portion of the stent graft was not fitted to the CCA lumen, and the contrast medium filled the stump. A self-expandable Precise stent (9 × 40 mm; Cordis, Miami, FL, USA) was deployed over the stent graft. After a Rider PTA catheter (8 × 40 mm; Leventon S.A., Barcelona, Spain) was expanded at the CCA, the contrast medium no longer filled the stump. Post-stenting DSA showed no stent thrombosis, but sluggish flow was observed in the left MCA. Diffusion-weighted MRI revealed new cortical embolisms in the left ACA and MCA territory. A neurologic examination revealed aggravation of the hemipareses (right arm, grade 3; right leg, grade 4; left leg, grade 2). The patient was on continuous anticoagulation and triple antiplatelet therapy, and was last seen 6 months after the procedure, at which point he remained with no new events and all of his pareses had only slightly improved (all grade 4).
A carotid stump is a rare source of microemboli, and all other possible sources of emboli must be excluded before assuming that the occluded ICA is the cause of symptoms (12345). In our patient, DSA excluded any atheromatous lesion in the aortic arch, CCA, and ECA and demonstrated a bulbous stump without trickling flow through ICA. Most of all, the patient was free from new events 6 months after exclusion of the stump.
Management of symptomatic ICA occlusion remains controversial. In a case with good retrograde filling of the distal ICA from the ophthalmic artery, anterograde ICA reopening may be a feasible option under balloon protection (67). However, exclusion of stump was performed because this lesion was regarded as chronic occlusion in stage and when a balloon protection device was not available.
For the exclusion of the stump, a self-expandable stent graft is the gold standard (345). Though a self-expandable stent graft was not available at the time of the procedure, we attempted to place a balloon-expandable stent graft. It was neglected that a balloon-expandable stent can be deformed by compression or cannot sufficiently be expanded, and in such a case, additional stenting is necessary. Contrary to the manufacturer's recommendations specifying that the introducer for a Jostent peripheral stent graft should be two sizes larger than the usual introducer size for balloon catheter, a 7-mm balloon catheter loading stent graft could not enter the 7-Fr. shuttle sheath. We switched to a 5-mm balloon catheter loading stent graft, which fitted the ECA lumen but not the CCA lumen. Considering that fitting to the CCA lumen without a gap is essential for the exclusion of a stump, an additional larger-caliber balloon or stent may be regarded as the feasible option. We thought that there would be a risk of rupture (9) or recoiling with additional dilatation of a larger-caliber balloon. Therefore, a self-expandable stent was placed to impose a consistent radial force on the stent graft and to resist collapse in the area of exposure to external compression (10). Final post-stent angioplasty with an 8-mm balloon alone, which can easily enter a 7-Fr. shuttle sheath, was performed at the CCA segment, resulting in both successful apposition to the CCA lumen and exclusion of the stump.
With the exception of a microembolism, the risk of complication was minimized, including vessel rupture during balloon expansion (9) and tissue necrosis from ECA sacrifice. In our patient, special care was taken to position the distal end of the stent graft to preserve the patency of as many ECA branches as possible. Only the superior thyroidal branch was sacrificed, and there was no associated complication. However, cerebral a microembolism via the retrograde collateral pathway of the ophthalmic artery was not prevented. The use of an endovascular protection device would have been helpful to prevent a microembolism, but it is likely that a microembolism was inevitable before placement of the protection device as a result of the repeated struggle to place a shuttle sheath over the acute angle between the arch and the CCA.
In conclusion, sequential self-expandable stent fitting into the CCA lumen upon balloon-expandable stent-graft fitting of the ECA lumen is a useful troubleshooting protocol for the successful exclusion of a symptomatic carotid stump when a self-expandable stent graft is not available and there is incompatibility between balloon loading stent graft and a 7-Fr. shuttle sheath.
Figures and Tables
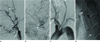
Fig. 1
Fig. 1. A 69-year-old man with a symptomatic carotid stump.
A. Left common carotid injection angiogram shows the occlusion of internal carotid artery (ICA) with stump (asterisk) and reconstituted ophthalmic flow into the distal ICA (arrowhead).
B. Retrograde filling (arrow) from the ophthalmic artery (arrowhead) supplies anterior cerebral circulation.
C. Arch aortogram reveals severe elongation of the aortic arch, making an acute angle with the common carotid artery. The left ICA is not seen with the remaining stump (asterisk).
D. Native radiography after a stent graft reveals the self-expandable stent (black arrows) within the stent graft (white arrows) and the excluded stump with contrast stasis (asterisk).
References
1. Barnett HJ, Peerless SJ, Kaufmann JC. "Stump" on internal carotid artery--a source for further cerebral embolic ischemia. Stroke. 1978; 9:448–456.
2. Kumar SM, Wang JC, Barry MC, Farrell L, Kelly CJ, Fitzgerald PH, et al. Carotid stump syndrome: outcome from surgical management. Eur J Vasc Endovasc Surg. 2001; 21:214–219.
3. Naylor AR, Bell PR, Bolia A. Endovascular treatment of carotid stump syndrome. J Vasc Surg. 2003; 38:593–595.
4. Nano G, Dalainas I, Casana R, Malacrida G, Tealdi DG. Endovascular treatment of the carotid stump syndrome. Cardiovasc Intervent Radiol. 2006; 29:140–142.
5. Carrafiello G, Delodovici ML, Piffaretti G, Castelli P. Endovascular treatment of carotid stump syndrome. Vasc Endovascular Surg. 2009; 43:277–279.
6. Suh DC, Kim JK, Choi CG, Kim SJ, Pyun HW, Ahn C, et al. Prognostic factors for neurologic outcome after endovascular revascularization of acute symptomatic occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 2007; 28:1167–1171.
7. Choi BS, Park JW, Shin JE, Lü PH, Kim JK, Kim SJ, et al. Outcome evaluation of carotid stenting in high-risk patients with symptomatic carotid near occlusion. Interv Neuroradiol. 2010; 16:309–316.
8. Macdonald S, Lee R, Williams R, Stansby G. Delphi Carotid Stenting Consensus Panel. Towards safer carotid artery stenting: a scoring system for anatomic suitability. Stroke. 2009; 40:1698–1703.
9. Broadbent LP, Moran CJ, Cross DT 3rd, Derdeyn CP. Management of ruptures complicating angioplasty and stenting of supraaortic arteries: report of two cases and a review of the literature. AJNR Am J Neuroradiol. 2003; 24:2057–2061.
10. Lownie SP, Pelz DM, Lee DH, Men S, Gulka I, Kalapos P. Efficacy of treatment of severe carotid bifurcation stenosis by using self-expanding stents without deliberate use of angioplasty balloons. AJNR Am J Neuroradiol. 2005; 26:1241–1248.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download