Abstract
Epithelioid hemangioendotheliomas (EHE) are uncommon and unpredictable vascular tumors that originate in skin, soft tissue, visceral organs, and bones. EHE occurring in the spine has been mostly reported as an osteolytic lesion. To the best of our knowledge, paravertebral EHE with sclerotic bone changes has not been reported in the literature. Here, we report a case of paravertebral EHE accompanied by adjacent sclerotic bone changes in the imaging findings on radiography, CT and magnetic resonance, as well as surgical and pathologic findings.
Epithelioid hemangioendothelioma (EHE) is a rare vascular tumor with a borderline malignant clinical outcome, which was first described by Weiss and Enzinger (1). EHE can arise from various sites including bone, liver, spleen, pleura, lung, skin, or soft tissue (2). Even though several cases of EHE in the spine have been reported, there are, so far as we know, no reports of it affecting adjacent spine with an ivory vertebra. We report here the illustrative imaging features of a paravertebral EHE associated with an ivory vertebra in a 46-year-old man. We believe that these findings may be helpful for the diagnosis of subsequent similar cases.
A 46-year-old man with chronic hepatitis underwent liver CT for further evaluation of a recent increase in serum α-FP. The CT incidentally showed a solid mass in the left anterior aspect of the paravertebral space at the level of the L2 vertebra. The patient reported that he had a mild pain on the left side of his waist for the last 13 years. He reported mild radiating pain and numbness in the anterior aspect of the left thigh. On physical examination, there was no direct or indirect tenderness, or a palpable mass. A neurologic examination found all findings to be normal. Radiography showed localized sclerotic changes in the L2 vertebral body (Fig. 1A). Three-dimensional multidetector CT imaging (LightSpeed; GE Healthcare, Milwaukee, WI, USA) was performed. Axial and coronal reformatted CT scans revealed a soft tissue mass that was about 3.1 × 3.2 × 3 cm in size in the left anterior aspect of the paravertebral space (Fig. 1B). The periphery of the mass was poorly defined and showed streaky or linear infiltrations into the adjacent tissue. Medially, the adjacent bony cortex of the L2 body showed mild scalloping in the region abutting the margin of the mass (Fig. 1C), and most of the L2 body showed dense reinforcement or diffuse sclerotic changes, that is, the "Ivory Vertebra Sign".
The MRI (MR Signa Excite; GE Healthcare, Milwaukee, WI, USA) demonstrated a distinct solid mass with low signal intensity on T1 weighted image (WI) and intermediate signal intensity on T2WI in the anterior aspect of the paravertebral space, which is also continuous with the anterolateral side of the left neural foramen at the level of L2-3 (Fig. 1C, D). Also, the left L2 spinal nerve was displaced and made contact with the mass. A dense sclerotic portion of the L2 body on CT showed intermediate signal intensity to slightly low signal intensity to that of the fat marrow on T1WI (Fig. 1C) and mild diffuse enhancement (Fig. 1E). The mass infiltrated the adjacent psoas muscle and caused pseudohypertrophic changes with fatty overgrowth in the left psoas muscle. The initial differential diagnosis included a slow growing benign tumor with reactive changes in the adjacent bone and muscle. A benign vascular tumor adjacent to the bone was the first consideration. A neurogenic tumor arising from the left L2 spinal nerve was included in the differential diagnosis due to the location of the mass. A healing stage with an organizing abscess and a juxtacortical soft tissue tumor containing a fibrous component or compact cellularity were considered due to the relatively low signal intensity on T2WI.
The patient underwent tumor excision. The tumor was a firm and yellowish mass adhesive to surrounding soft tissue, but the outer margin of the tumor was relatively well-demarcated. It was difficult to differentiate the mass from the left L2 spinal nerve because of their adhesion. Hence, careful microscopic resection of the tumor from surrounding soft tissue was performed and enucleation of the tumor was relatively easier than expected. On gross examination, the excised mass was a soft gray tan tissue on the surface. Light microscopy disclosed trabeculated or cord-like nests of tumor cells within the myxohyalinized stroma with degeneration (Fig. 1F). Some of the tumor cells had intracytoplasmic lumina, which occasionally showed intraluminal red blood cells. The nuclei of epithelioid tumor cells were vesicular, round to oval, but occasionally indented. Lymphoplasmacytic infiltration was noted in adipose tissue and the degenerated muscle fibers near the tumor (Fig. 1G). Tumor cells were stained with CD31 (Fig. 1H), CD34 and factor VIII, which is indicative of a vascular tumor, and were not stained with EMA, S-100, SMA, cytokeratin and CD45, which is indicative of a meningioma, neurogenic tumor, inflammatory myofibroblastic tumor, metastatic carcinoma and malignant lymphoma, respectively. Morphologic and immunohistochemical findings were consistent with epithelioid hemangioendothelioma.
EHE is a distinctive vascular tumor presenting with biologic behavior intermediate between a hemangioma and a conventional angiosarcoma. These tumors have the ability to recur locally and metastasize. EHE can occur at any anatomic location, including the liver, lung, bone, brain, lymph node, oral cavity, stomach, mediastinum, spleen, heart, and skin. EHE of lung, liver, and bone is frequently multicentric while a soft tissue lesion is usually solitary. EHE of soft tissues shows no sex predilection, and is seen mainly in middle aged adults, whereas cases in children are extremely rare.
In our case, a juxtacortical mass at the corner of the L2 body was located in the left anterior aspect of the paravertebral space. The L2 body beside the ovoid solid mass showed an ivory vertebra. The differential diagnosis of an ivory vertebra associated with a paravertebral mass usually includes a lymphoma, osteoblastic metastasis, osteomyelitis, and so on (3). An ivory vertebra associated with a paravertebral vascular mass has not been reported, on the other hand, EHE associated with osteolytic changes in the spine has been reported on several case reports (456 78). In our case, the sclerotic zone of the L2 body on CT showed the mixed signal intensity including the fat signal on T1WI and markedly decreased enhancement compared to the paravertebral enhancing mass on gadolinium-enhanced fat suppressed T1WI, so differently that the common diseases associated with an ivory vertebra show low signal intensity on T1WI and similar enhancement to the paravertebral mass. Also, the outer surface of the L2 body adjacent to the mass was scalloped with a mild degree of sclerosis, indicating chronic irritation. Although reactive bone changes adjacent deep soft tissue vascular tumor of the extremities occurred in about 21% of cases, sclerotic change associated with a vascular tumor is very rare. Sclerosis among their bone change was cortical thickening by periosteal reactions (9). Similar to the reactive cortical sclerosis of the juxtracortical hemangioma in the tubular bone of the extremity (10), the ivory vertebra of our case would seem to be a reactive change, which is cortical and trabecular sclerosis in the flat bone of the spine. Sclerotic bone change adjacent to the mass presumably is secondary either to stretching or irritation of the periosteum, or to local hyperemia caused by the hypervascular lesion (10). Although we could not get tissues of the vertebral body, sclerotic bone changes in the vertebral body are thought to be a reactive bone formation considering that microscopic findings of adipose tissue and muscle fibers near the tumor showed only inflammatory infiltration instead of tumor cells but discernible inflammatory cell infiltration was absent within the tumor. In addition, imaging findings of the vertebral body which showed distinguishing signal intensity through T1 shortening, less prominent enhancement and extrinsic indentation on surface of the body are different from those of the tumor. Our case is the first case, as far as we know, of a paravertebral EHE that elicited an ivory vertebra.
Nonvascular components can also be seen in angiomatous lesions including fat, smooth muscle, fibrous tissue, bone, hemosiderin and thrombus, which show variable signal intensities on MRI. Among the nonvascular components, fat overgrowth is the most common finding. If the lesion has fat, hemorrhage or calcification causing a T1 shortening effect, it is likely to be a benign tumor. But in our case, the juxtacortical solid mass did not show T1 shortening effect. MRI showed fat overgrowth in the psoas muscle near the mass; this not a nonvascular component of angiomatous tumor but a reactive change as microscopically confirmed mature adipocytes.
Because of the extraforaminal tumor location and the poorly defined L2 spinal nerve that was inseparable from the tumor, we also considered a neurogenic tumor. Because EHE often tends to involve neurovascular structures, it can clinically mimic a nerve sheath tumor. But the imaging finding with infiltrative margins and reactive changes in the adjacent tissue was not matched to the neurogenic tumor.
The signal intensity of the mass on T2-weigted magnetic resonance (MR) images was not as strong as that of typical soft tissue tumors. This distinctive signal intensity lesion can be seen in a variety of tumors containing compact cellularity, collagenous or mature fibrous tissue, high flow vascularity or hemorrhagic foci among other possibilities. This is nonspecific as with many other diseases, but is consistent in our case.
In this report, imaging features on radiography, CT, MR and clinicopathologic findings in the paravertebral EHE with an ivory vertebra which is an unusual condition were presented. We expect that these findings may be helpful for the diagnosis of subsequent similar cases.
Figures and Tables
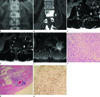
Fig. 1
A 43-year-old man with paravertebral epithelioid hemangioendothelioma.
Radiograph with anteroposterior view (A) reveals uniformly increased opacity in the left side of L2 body. Coronal reformatted CT image (B) for the L2 vertebra in the bone window reveals homogeneous increased opacity involving nearly the entire vertebral body. Focal scalloping with sclerotic bony changes in the cortex of L2 is noted in the region abutting the margin of the soft tissue mass (star).
MRI with axial T1 weighted image (WI) (C) and T2WI (D) shows a paravertebral solid mass (star) with infiltrative margins and adjacent fat proliferation in the psoas muscle (solid white arrows). A dense radiopaque area on CT shows a mixed signal intensity containing the fat signal (dashed white arrows).
The coronal scan with T1WI depicts the relationship of the mass and left L2 spinal nerve (not shown). After gadolinium administration, fat-suppressed T1WI (E) shows intense diffuse enhancement in the mass and mild enhancement in the L2 vertebra and left psoas muscle. Photomicrograph (F) of the specimen shows that the tumor cells are arranged in cord-like pattern within myxohyalinized stroma (Hematoxylin & Eosin, × 200).
Photomicrography (G) of the specimen near the tumor shows infiltration of lymphoplasma cells to adipose tissue outside the tumor (× 100). Degenerated muscle fibers that mature adiopocytes and inflammatory cells infiltrate are noted near the tumor. A few muscle fibers are hyalinized and necrotic.
Immunohistochemical stain (H) shows that tumor cells were stained with CD31, a representative marker of vascular endothelial cells (× 200).
References
1. Weiss SW, Enzinger FM. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982; 50:970–981.
2. Weiss SW, Goldblum JR. Hemangioendothelioma: vascular tumors of intermediate malignancy. In : Weiss SW, Goldblum JR, editors. Enzinger and Weiss's Soft tissue tumors text book. Philadelphia: MosbyElsvier;2008. p. 681–702.
3. Graham TS. The ivory vertebra sign. Radiology. 2005; 235:614–615.
4. Gokhan GA, Akyuz M, Gurer IE, Tuncer R. Epithelioid hemangioendothelioma derived from the spine region: case report and review of the literature. Wien Klin Wochenschr. 2006; 118:358–361.
5. Christodoulou A, Symeonidis PD, Kapoutsis D, Iordanidis F. Primary epithelioid hemangioendothelioma of the lumbar spine. Spine J. 2008; 8:385–390.
6. Abrahams TG, Bula W, Jones M. Epithelioid hemangioendothelioma of bone. A report of two cases and review of the literature. Skeletal Radiol. 1992; 21:509–551.
7. Ellis TS, Schwartz A, Starr JK, Riedel CJ. Epithelioid hemangioendothelioma of the lumbar vertebral column: case report and review of literature. Neurosurgery. 1996; 38:402–407.
8. Sirikulchayanonta V, Jinawath A, Jaovisidha S. Epithelioid hemangioma involving three contiguous bones: a case report with a review of the literature. Korean J Radiol. 2010; 11:692–696.
9. Sung MS, Kang HS, Lee HG. Regional bone changes in deep soft tissue hemangiomas: radiographic and MR features. Skeletal Radiol. 1998; 27:205–210.
10. Yao L, Lee JK. Case report 494: hemangioma of surface of ulna with prominent sclerosis. Skeletal Radiol. 1988; 17:378–381.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download