Abstract
Purpose
The purpose of this study is to determine the characteristic dynamic enhancement pattern of magnetic resonance (MR) imaging for malignant thyroid tumor.
Materials and Methods
Eight patients who were pathology proven to have a malignant thyroid tumor, preoperatively. There are 5 papillary carcinomas, 1 medullary carcinoma, 1 follicular carcinoma, and 1 fine needle aspiration biopsy proven atypical cell.
Results
Based on preoperative MR imaging, we compared the dynamic MR enhancement pattern relating to the pathologic type. On contrast agent-enhanced dynamic T1-weighted image (T1WI), 5 papillary carcinoma and one medullary carcinoma showed delayed enhancement compared to normal parenchyma. In addition, one follicular carcinoma shows stronger enhancement than normal parenchyma, with one papillary carcinoma showing a persistent decrease in enhancement compared to normal parenchyma.
Conclusion
Although this study is limited by a small patients population, the data suggests that delayed enhancement on enhanced dynamic T1WI is a possible characteristic MR finding of a malignant thyroid tumor. I think that the comparison of MR imaging between benign and malignant nodules is required for a correct characterization.
Most thyroid diseases are evaluated by radionuclide scintigraphy, sonography or percutaneous needle biopsy. Sonography is perhaps the most definitive method for evaluation of nodules in a thyroid gland. However, the disadvantages of this technique include limited penetration in some anatomic locations, such as retrotracheal and substernal areas, and a limited field of view for assessing large masses. Further, current diagnostic methods may not enable the accurate detection of the malignant nature of a thyroid nodule, especially in a multinodular goiter.
Therefore, new techniques are being investigated to improve the evaluation of thyroid nodules.
The magnetic resonance (MR) imaging has a minor role in the evaluation of most patients with thyroid nodules or goiters, because of inadequate spatial and contrast resolution and only a few cases documented. However, technological advances have provided good spatial and contrast resolution in the neck including the thyroid gland, parathyroid gland and lymph node. Also it has made available the dynamic keyhole imaging of the thyroid nodule with spin echo sequences.
The aim of our study was to evaluate this new technique in the routine evaluation of possible thyroid cancer, and particularly for the determination of the characteristic MR dynamic enhancement pattern of a malignant thyroid tumor.
This study series included eight patients (1 male and 7 females; mean age: 56 years; age range: 22-81 years) who were pathologically proven to have a malignant thyroid tumor, preoperatively; 5 papillary carcinoma, 1 medullary carcinoma, 1 follicular carcinoma, and 1 fine needle aspiration biopsy (FNAB) proven atypical cell (with multiple metastatic lymph nodes in the neck). Preoperative MRI images had been obtained in all patients.
We got the approval from the Institutional review board and informed consent from each patient part of this study. A pathologic report and ultrasonography (USG) findings were collected retrospectively.
All images were obtained with a 3.0-T MR imaging unit, Magnetom Verio (Siemens Medical Systems, Erlangen, Germany).
The MRI protocol was constituted of pulse sequences including an axial fast spin-echo T2-weighted image (T2WI), axial T1-weighted image (T1WI), and contrast agent-enhanced dynamic T1WI.
1) The axial fast spin-echo T2WI was obtained in approximately 3 minutes, 41 seconds. Imaging was performed in the 30 axial planes with 3-mm slice thickness, and no interslice gap. The field of view was 140 cm and the voxel size was 0.4 × 0.4 × 3.0 mm. The T2WI were acquired with a repetition time (TR) of 4,100 ms and an echo time (TE) of 137 ms. The flip angle was 90 degrees.
2) The axial T1WI was obtained in approximately 6 minutes, 57 seconds. Imaging was performed in the 30 axial planes with a 3-mm slice thickness, and no interslice gap. The field of view was 140 cm and the voxel size was 0.5 × 0.5 × 3.0 mm. The T1WI were acquired with a TR of 2,740 msec and an TE of 48 msec. The flip angle was 70 degrees.
3) The dynamic spin-echo sequence was a routine axial T1 spin-echo sequence obtained during contrast infusion.
The image was obtained in approximately 9 minutes, 13 seconds. Imaging was performed in the 88 axial planes with a 0.7-mm slice thickness and no interslice gap. Slice oversampling was 36.4%. The field of view (read/phase) was 255 mm/50.0%, and the voxel size was 0.7 × 0.5 × 0.7 mm. The images were acquired with a TR of 5.12 msec and an TE of 1.99 msec. The flip angle was 10 degrees and time to center was 26.4 sec.
Contrast enhanced dynamic T1WI were obtained immediately after injection (as a precontrast scan) and at 1 min 30 sec/3 min/4 min 30 sec/6 min after injection of Gadovist.
Paramagnetic contrast agent (gadobutrol, Gadovist; Schering, Berlin, Germany) was administered intravenously at a weight-adjusted dose of 0.15 mmol per kilogram of patient body weight.
Based on preoperative MR imaging, we compared the dynamic MR enhancement pattern relating to the pathologic type. All biopsy-proven malignant thyroid tumors showed hypoechogenicity on previous USG, except for one follicular carcinoma (isoechogenicity). In addition, only one patient with papillary carcinoma remained on the color Doppler USG. In this case, the malignant nodules showed decreased vascularity compared to normal parenchyma.
1) On a T1WI, one papillary carcinoma showed high signal intensity (SI) and one medullary carcinoma showed low SI compared to normal parenchyma. And, other cases were not differentiated compared to normal parenchyma.
2) On a T2WI, 3 papillary carcinomas and one follicular carcinoma showed high SI, and one papillary carcinoma showed low SI. The other case was not differentiated with normal parenchyma.
3) On contrast agent-enhanced dynamic T1WI, 4 papillary carcinomas and one medullary carcinoma, as well as 1 FNAB proven atypical cell (Fig. 1) showed delayed enhancement compared to normal parenchyma. And, one follicular carcinoma (Fig. 2) showed stronger enhancement than normal parenchyma, and one papillary carcinoma (Fig. 3) shows persistently decreased enhancement compared to normal parenchyma.
About 75% of all cases showed delayed enhancement pattern on contrast-enhanced dynamic T1WI (Table 1).
Our study found that the maximum gland-lesion contrast difference occurred 3 to 4 minutes and 30 seconds after contrast infusion.
In addition, two patients with benign nodules (nodular hyperplasia) underwent the same MR imaging. One patient had a homogeneous solid nodule on USG. On contrast agent-enhanced dynamic T1WI, the nodule shows nearly the same enhancement as normal parenchyma. Other patients had heterogeneous solid and cystic nodules on USG. These nodules also had a hemorrhagic portion on preT1WI. On contrast agent-enhanced dynamic T1WI, the nodules show heterogeneous enhancement, and some part of the nodules showed nearly the same enhancement as normal parenchyma and other parts showed early or delayed enhancement compared to parenchyma.
Although this study has the limitation of a small patient population, the data suggest that delayed enhancement on contrast enhanced dynamic T1WI is a possible characteristic MR findings of malignant thyroid tumor. The delayed enhancement pattern of thyroid cancer on dynamic T1WI is similar to that of pituitary adenoma. We hypothesize that the enhancement pattern of these tumors is related with tumor angiogenesis and its vascularity. Pituitary tumors are known to be less vascular than normal pituitary tissue, suggesting that angiogenesis may be inhibited in these tumors. This is consistent with the findings of Jugenburg and colleagues, but is in marked contrast to studies in other tissues including the breast, prostate, and lung, in which tumors are more vascular than the respective normal (3). In contrast to the majority of solid tumors, pituitary adenomas show a decreased expression of vascular endothelial growth factor and have significantly lower microvessel densities compared to non-neoplastic pituitary glands (4). Similarly, the microvascular density was higher in normal tissue than in benign and malignant proliferative lesions of the thyroid gland (1). Microvessel density is a surrogate marker of tumoral angiogenesis. Microvessel density assessment is the most commonly used technique to quantify intratumoral angiogenesis in cancer. It was first developed by Weidner et al. (7) and used for panendothelial immunohistochemical staining of blood microvessels (8). In contrast to thyroid tumor and pituitary adenoma, hypervascular tumors such as breast and prostate cancer, shows early enhancement pattern on dynamic T1WI.
In addition, breast and prostate MR imaging is being widely used with increasing frequency to aid in the detection and evaluation of breast and prostate malignancies. We explained that, contrary to common cancers, but similar to other endocrine neoplasms such as pituitary and adrenal cortex tumours, angiogenesis is reduced in some thyroid proliferative lesions compared with normal tissue (1, 2). So, thyroid cancer shows a delayed enhancement pattern on contrast enhanced dynamic T1WI, probably due to its hypovascularity.
Two cases did not show a delayed enhancement pattern on dynamic T1WI. One case of follicular carcinoma showed strong early and persistent enhancement on contrast-enhanced dynamic T1WI, compared to normal parenchyma. This nodule is about a 3 cm sized lesion, the largest identified in our study. So, relatively well developed vascularity within the large cancer caused a different dynamic enhancement pattern. This patient had hashimoto thyroiditis, which could also explain the unknown underlying parenchymal change in the thyroid gland enhancement pattern. The other case of papillary carcinoma showed a persistently decreased enhancement pattern on dynamic T1WI, compared to normal parenchyma. The nodule was about a 5 mm sized lesion, relatively small compared to among the patients. Also this nodule has internal punctate tiny echogenicity, suggesting a microcalcification on USG. Hence, a relatively poorly developed vascularity within the small cancer caused a different dynamic enhancement pattern. Much to our regret, only one case of papillary carcinoma remained in the color Doppler USG. And, this malignant nodule shows hypovascularity compared to the parenchyma on USG and also showed a delayed enhancement pattern on the dynamic enhanced T1WI. But, because of the other 7 cases had not remained on the Doppler USG, it was difficult to perform a correlation analysis between Doppler USG and dynamic enhanced MR imaging in our study. We found that the maximum gland-lesion contrast difference peaked at 3 minutes to 4 minutes, 30 seconds after contrast infusion, suggesting that this is the most sensitive time for detecting lesions of the thyroid gland. Our dynamic technique was easy to perform and did not add a time penalty to the pituitary study. The practice of examining the gland twice, once during and after contrast infusion, was effective. In conclusion, delayed enhancement on contrast-enhanced dynamic T1WI is a possible characteristic MR finding of malignant thyroid tumors. Also, dynamic contrast-enhanced magnetic resonance imaging can be useful to detect or exclude thyroid carcinoma with high diagnostic accuracy in patients with a multinodular goiter when results of other diagnostic methods are inconclusive. The previous early results with MR of the thyroid indicate inadequate spatial and excellent contrast resolution for depicting disease in these glands (5, 6). The tool for the diagnosis of thyroid will be tempered by the traditional reliance on scintigraphy and sonography for this purpose. However, with the development of the MR technique, MR imaging has been shown to be a promising technique for imaging the thyroid. Moreover clear indications for MR in the evaluation of these glands are emerging. The limitations of our study was the relatively small patient population and the fact that we did not compare the benign and malignant thyroid lesions. Future studies should compare the MR imaging between benign and malignant nodules for the correct characterization of the tumor.
Figures and Tables
Fig. 1
On ultrasonography, an approximately 8 mm, well define hypoechoic nodule is seen in the mid level of the left lobe of the thyroid gland. This is dynamic images of the thyroid gland in the axial section. The nodule (arrow) was not clearly delineated immediately after contrast injection (as a precontrast scan). Early-phase dynamic images obtained 1 minute 30 seconds after injection best depicts the nodule (arrow) in left thyroid gland. The nodules show decreased enhancment in the early phase of a dynamic enhacement study, compared to normal parenchyma. In addition, the nodule (arrow) shows gradually centrifetal delayed enhancement. A 6-minute image after contrast injection indicates that the nodule is masked into the backgronund thyroid tissue.
Note.-FNAB = fine needle aspiration biopsy
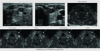
Fig. 2
On ultrasonography, an approximately 3 centimeter, well defined hyperchoic nodule (white arrow) is seen in right lobe of the thyroid gland at the mid level. On a dynamic image obtained 3 minutes after injection, the nodule (white arrow) shows strong early enhancement compared to the parenchyma of the left lobe. In addition, the nodule (white arrow) shows persistent well enhancement, compared to normal parenchyma. In a 6-minute image after contrast injection, the nodule (white arrow) shows persistent enhancement, but background parenchyma is washed out.

Fig. 3
On ultrasonography, an approximately 5 millimeter, well hypoechoic nodule (arrow) is seen in the right lobe of the thyroid gland at mid level. This nodule has internal punctate tiny echogenicity, suggesting a tiny calcification. On a dynamic enhancement study, the nodule (arrow) shows a persistent decrease in enhancement compared to background parenchyma.

References
1. de la Torre NG, Buley I, Wass JA, Turner HE. Angiogenesis and lymphangiogenesis in thyroid proliferative lesions: relationship to type and tumour behaviour. Endocr Relat Cancer. 2006; 13:931–944.
2. Jugenburg M, Kovacs K, Stefaneanu L, Scheithauer BW. Vasculature in nontumorous hypophyses, pituitary adenomas, and carcinomas: a quantitative morphologic study. Endocr Pathol. 1995; 6:115–124.
3. Turner HE, Nagy Z, Gatter KC, Esiri MM, Harris AL, Wass JA. Angiogenesis in pituitary adenomas and the normal pituitary gland. J Clin Endocrinol Metab. 2000; 85:1159–1162.
4. Niveiro M, Aranda FI, Peiró G, Alenda C, Picó A. Immunohistochemical analysis of tumor angiogenic factors in human pituitary adenomas. Hum Pathol. 2005; 36:1090–1095.
5. Stark DD, Moss AA, Gamsu G, Clark OH, Gooding GA, Webb WR. Magnetic resonance imaging of the neck. Part II: pathologic findings. Radiology. 1984; 150:455–461.
6. Higgins CB, McNamara MT, Fisher MR, Clark OH. MR imaging of the thyroid. AJR Am J Roentgenol. 1986; 147:1255–1261.
7. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991; 324:1–8.
8. Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, et al. Microvessel density and VEGF expression are prognostic factors in colorectal cancer Meta-analysis of the literature. Br J Cancer. 2006; 94:1823–1832.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download