Abstract
We present a case of scalp arteriovenous malformation (AVM) in a patient with severe hemophilia A. The 22-year-old man presented with a pulsatile right parietal scalp mass. Digital subtraction angiography revealed an AVM in the right parietal scalp, supplied by superficial temporal and occipital arteries that drained into multiple venous structures. We successfully performed direct puncture embolization followed by surgical resection of the scalp AVM in conjunction with supplemental infusion of coagulation factor VIII before, during and after the embolization and the operation.
An scalp arteriovenous malformation (AVM) is an abnormal fistulous connection between feeding arteries and draining veins without an intervening capillary bed within the subcutaneous layer. The draining veins are typically grossly dilated and may show variceal dilation that is not life threatening, but can cause cosmetic deformity (1). Hemophilia A, transmitted as an X-linked recessive trait, is a common heritable bleeding disorder caused by mutations in the gene that codes for factor VIII (2). Here, we describe an unusual case of scalp AVM in a patient with severe hemophilia A whom we treated with direct puncture embolization using coils and n-butyl-cyanoacrylate (NBCA), followed by surgical removal.
A 22-year-old man presented with a pulsatile mass on his right parietal scalp at birth and had increased in size during the preceding year. The patient had no previous history of trauma or head injury. Moreover, he was previously diagnosed with hemophilia A and hemophilic arthropathy, and had undergone surgery for hemophilic arthropathy of the left knee and left elbow joints 7 years prior to presentation. His plasma factor VIII was less than 1%, indicating severe hemophilia A (normal range, 50-80%). His prothrombin time was 12.2 seconds (control, 12.5-14.7) and his activated partial thromboplastin time was 59 seconds (control, 29-43). Upon examination, we observed no ulceration of the scalp skin or active bleeding.
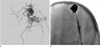
Enhanced computed tomography demonstrated prominent vascular enhancement with an aneurysmal sac on the right parietal scalp (Fig. 1). Digital subtraction angiography (DSA) was performed to evaluate the scalp mass and to plan treatment. To ensure effective hemostasis for intra-arterial catheterization, a bolus dose of coagulation factor VIII (4500 units) [Greenmono, monoclonal anti-FVIII antibodies (mAb) supplied by Hyland Division, Baxter Healthcare Corp., Deerfield, IL, USA] was administered before angiography. DSA showed that the superficial temporal and occipital arteries supplying the nidus were dilated, along with the multiple dilated early drained venous structures and a dilated aneurysmal pouch (Fig. 2). There were no bleeding complications at the puncture. Complete removal of the scalp AVM was planned because severe hemophilia A can lead to serious and life-threatening bleeding, even during minor trauma.
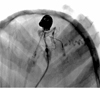
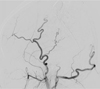
Eight days after the initial angiography, preoperative percutaneous embolization of the scalp AVM was performed immediately after intravenous injection of coagulation factor VIII. Initially, the right common femoral artery was punctured and a 6 Fr sheath was inserted into the right femoral artery, followed by a 5 Fr Berenstein catheter inserted into the right external cerebral artery (ECA) for control angiography. The dilated aneurysmal sac near the fistulous or arteriovenous connection point was punctured by an 18G angiocath (Fig. 3A). Pushable detachable coils (Nester 14 mm/12 cm × 7, 14/10 × 2, 14/8 × 1, 14/6 × 2, 14/4 × 1 and Tornado coils 4 mm/2 cm × 2) were inserted into the aneurysmal sac to obliterate the arterio venous (AV) shunting and nidus (Fig. 3B). Coil embolization of the scalp AVM was attempted before NBCA injection because the nidus and distal venous structural sac were too large to treat with liquid material such as NBCA, and additionally, there were too many feeding arteries and draining veins of the AVM. DSA of the right ECA showed decreases in flow and shunting, but still contained some shunting and dilated venous structures. A new nidal point puncture was attempted and two sessions of 25% NBCA injection with lipiodol were performed under manual compression of the draining veins (Fig. 4). Right ECA angiography after percutaneous embolization showed complete obliteration of the AVM nidus and arteriovenous shunts (Fig. 5). A small amount of glue was administerd into the draining veins and feeders. There was no significant bleeding during or after these procedures.
Two weeks after percutaneous embolization, enucleation of the scalp AVM was performed because the AVM had decreased in size after coiling with glue injection, but still retained mass formation and cosmetic problems. A bolus dose of 4,500 units of coagulation factor VIII was administered before surgery. During the operation, 4,500 units were administered daily by continuous intravenous infusion. The embolized AVM was safely removed by surgery without any morbidity. After the operation, the patient received coagulation factor VIII (1,500 units, tid). We maintained FVIII levels > 80% for 4 days. To assist with the administration of coagulation factor VIII, laboratory tests (aPTT, PT, FVIII level, platelet count) were performed on a daily basis. The patient was discharged after 4 days.
Scalp AVM is a relatively rare type of vascular lesion and usually gradually increases in size from birth. In this report, the patient complained of a recent increase in the size of the mass, which resulted in cosmetic problems. Rapid increases in size have been reported to occur at puberty, during pregnancy and during menstruation (1). Other symptoms and signs of scalp AVM include pain, headaches, swelling of soft tissue, changes in the skin and bruit. It can even cause scalp necrosis and bleeding (3). High output cardiac failure can occur with large fistulae (1).
Treatment of scalp AVM is generally considered when complications arise, including bleeding, ulceration, high output cardiac failure, and cosmetic disfiguration. Treatment options for scalp AVM include surgical treatment, endovascular treatment, or a combination of both (34). In the past, surgical excision or ligation of feeding arteries was the treatment of choice for scalp AVM. However, with the advent of endovascular treatment techniques and new embolic agents, embolization has become the preferred treatment for these lesions. There are three endovascular treatments for scalp AVM: transarterial, transvenous and direct puncture embolization (5). The risk of necrosis of the overlying skin may be increased due to the embolization of bilateral superficial temporal arteries when the lesion is in or near the midline. In such situations, direct puncture embolization with NBCA, alcohol, or metallic coils is preferred. In direct puncture embolization, the venous structure just distal to the AV connection is targeted.
We report a patient with severe hemophilia A, whose plasma coagulation factor was less than 1% of the normal value. Because scalp AVM in patients with severe hemophilia A is relatively rare, there are no standard treatment options. Although embolization using metallic coils or liquid material has achieved successful results in selected cases, it usually results in palliation rather than cure (6). If surgical resection alone is used for AVM in hemophilia patients, patients often suffer from recurrent AVM or bleeding during the operation, as well as potential cosmetic morbidity (67). Therefore, preoperative embolization with surgical resection has been the main treatment option for scalp AVM with hemophilia.
In cases of AVM with hemophilia, preoperative transarterial embolization of the multiple feeding arteries may be ineffective or technically difficult because of the bleeding tendency. Without superselection of the nidus and supplying vessels, transarterial embolization often results in the occlusion of proximal arteries to the AVM and the development of prominent collateral vessels. To prevent this, the AV connection must be occluded by deep penetration of small particles or a liquid embolic agent such as NBCA by means of direct puncture of the nidus or venous pouch (5). Metallic coil embolization can prevent the spread of the liquid agent and decrease the size of AVM when performed before liquid embolic agent embolization (5). Scalp AVMs are less likely to communicate with the deep venous system because of their superficial locations. For these reasons, we performed manual compressions of venous drainage during glue injection to reduce undesirable washout into the distal venous outflow. To avoid exposure of the operator's hand to radiation or temporary occlusion of the venous outflow, a ring-shaped compression device can be used. But in case of an AVM with complex venous drainage, complete occlusion of the venous outflow with a simple device is difficult (58).
Scalp AVMs rarely induce serious hemorrhage, but minor trauma may result in serious complication such as life-threatening bleeding in hemophilic patients. Modern therapy, which incorporates differentiated coagulation factor replacement, makes it possible to perform major surgery on hemophilic patients with virtually no complications. Benndorf et al. (9) reported an unusual case of a mandibular AVM in a patient with severe hemophilia A and recommended endovascular treatment in mandibular AVMs, in which replacement therapy with coagulation factors enabled safe endovascular treatment in hemophiliac patients. In previous studies, including our case, hemophilia and hemostasis were achieved by either intermittent bolus injection or continuous intravenous infusion of coagulation factor.
In conclusion, replacement therapy that includes normal levels of coagulation factor enables the safe and effective endovascular treatment of scalp AVM by direct puncture embolization in hemophiliac patients.
Figures and Tables
Fig. 1
Contrast enhanced CT reveals prominent vessels with an aneurysmal sac on the subcutaneous layer of the right parietal scalp.
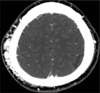
Fig. 2
Lateral projection images from right (A) and left (B) ECA angiography show opacification of an AVM of the right parietal scalp. The lesion was supplied by dilated superficial temporal and occipital arteries and were drained into multiple early dilated venous structures.
Note.-AVM = arteriovenous malformation, ECA = external cerebral artery

Fig. 3
A. Lateral projection from the direct puncture angiography shows filling of the lesion with contrast material and the venous drainage. B. Lateral projection after insertion of detachable coils (Nester and Tornado coils) shows multiple coils filling the aneurysmal sac and vascular structures.
References
1. Fisher-Jeffes ND, Domingo Z, Madden M, de Villiers JC. Arteriovenous malformations of the scalp. Neurosurgery. 1995; 36:656–660. discussion 660.
2. Bowen DJ. Haemophilia A and haemophilia B: molecular insights. Mol Pathol. 2002; 55:1–18.
3. Nagasaka S, Fukushima T, Goto K, Ohjimi H, Iwabuchi S, Maehara F. Treatment of scalp arteriovenous malformation. Neurosurgery. 1996; 38:671–677. discussion 677.
4. Jeong HS, Choi JY, Lee HJ, Kim TW, Kim MB, So YK, et al. Diagnosis and Treatment of Extracranial Arteriovenous Malformations in the Head and Neck Region. Korean J Otolaryngol-Head Neck Surg. 2005; 48:1136–1142.
5. Han MH, Seong SO, Kim HD, Chang KH, Yeon KM, Han MC. Craniofacial arteriovenous malformation: preoperative embolization with direct puncture and injection of n-butyl cyanoacrylate. Radiology. 1999; 211:661–666.
6. Jeong HS, Baek CH, Son YI, Kim TW, Lee BB, Byun HS. Treatment for extracranial arteriovenous malformations of the head and neck. Acta Otolaryngol. 2006; 126:295–300.
7. Widlus DM, Murray RR, White RI Jr, Osterman FA Jr, Schreiber ER, Satre RW, et al. Congenital arteriovenous malformations: tailored embolotherapy. Radiology. 1988; 169:511–516.
8. Ryu CW, Whang SM, Suh DC, Kim SM, Jang YJ, Kim HJ, et al. Percutaneous direct puncture glue embolization of high-flow craniofacial arteriovenous lesions: a new circular ring compression device with a beveled edge. AJNR Am J Neuroradiol. 2007; 28:528–530.
9. Benndorf G, Kim DM, Menneking H, Klein M. Endovascular management of a mandibular arteriovenous malformation in a patient with severe hemophilia a. AJNR Am J Neuroradiol. 2004; 25:614–617.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download