Abstract
Purpose
Materials and Methods
Results
Figures and Tables
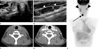 | Fig. 1An 18-year-old girl with papillary thyroid cancer presenting with a palpable neck mass. BRAFV600E mutation analysis was positive.Transverse (A) gray-scale US images depict a typical malignant nodule with an irregular margin, marked hypoechogenicity with microcalcifications, and taller than wide shape with metastatic lymph nodes on the right at level III (B) (arrowheads).
The pre (C) and post contrast-enhanced (D) neck CT scan show a slightly enhancing low attenuated nodule of the right thyroid gland.
The PET-CT scan (E) demonstrates a hypermetabolic nodule in the right lobe p-SUV 8.9 (arrow) and lymph node metastases (arrowheads). Histopathology of these lymph nodes show metastasis.
Note.-PET-CT = positron emission tomography-computed tomography
|
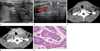 | Fig. 2A 13-year-old girl with papillary thyroid cancer.Transverse (A) gray-scale US images depict goitrous enlargement with numerous microcalcifications (arrow), (B) metastatic lymph node with calcifications and hypervascularity (arrowheads).
The pre (C) and post (D) contrast-enhanced neck CT scan show diffuse heterogeneous enlargement of the thyroid gland with calcifications, a metastatic enhancing mass in the strap muscle, and both level IV nodes.
Histologic examination (E) shows classical papillary thyroid cancer with optically clear nuclei and nuclear pseudoinclusions (H & E, × 400).
|
Table 1
Summary of Clinical Information in 13 Patients with Thyroid Cancer

Note.-*by the AJCC cancer staging manual and hand book, 7th Ed.
cTNM = clinical staging.
pTNM = pathological staging.
+ = mutation detected.
- = no mutation detected.
BRAFV600E mutation = B type raf kinase, CND = central neck dissection, HT = hemithyroidectomy, FNA = fine needle aspiration, FND = functional neck dissection, FTC = follicular carcinoma, minimal invasive, MRND = modified radical neck dissection, ND = neck dissection, NR = node resection, NT = not taken, PTC = papillary carcinoma, RET oncogene = rearranged during transfection, SND = selective neck dissection, TNM = tumor node metastasis, TT = total thyroidectomy, US = ultrasonography
Table 3
Summary of CT Features in 12 Children and Adolescents with Thyroid Cancer

Note.-*compared with to normal surrounding thyroid tissue.
†diffusely low attenuation of the thyroid gland, similar with to strap muscle on a precontrast scan due to underlying with lymphocytic thyroiditis; in these patients, attenuation of the nodules was is compared with to the neck muscle.
FTC = follicular carcinoma, PTC = papillary carcinoma




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download