Abstract
Synovial sarcoma is an uncommon soft tissue malignancy usually arising in the extremities of young adults. Synovial sarcomas at unusual anatomic locations have been reported; however, to the best of our knowledge, there are no reports on primary synovial sarcoma in the rectovesical space. Here, we describe the radiologic findings of primary synovial sarcoma in the rectovesical space and review relevant literature.
Synovial sarcoma is the fourth most common type of soft tissue sarcoma (1). It most commonly arises in the extremities and other locations, but in rare cases can arise in the extraperitoneal space (2). Most extraperitoneal synovial sarcomas that were reported arose in the abdominal retroperitoneum (3). Here, we report a case of primary synovial sarcoma arising in the rectovesical space. We present the radiological findings of this disease and discuss the relevant literature review.
A 48-year-old man presented with constipation and urinary symptoms such as frequency and urgency for a month. On a physical examination, abdominal distension was noted and a mass-like lesion was palpable anteriorly by digital rectal examination. Laboratory findings were normal. Computed tomography (CT) revealed a large soft tissue mass measuring 10 cm in a diameter in the pelvic cavity. The mass had a well-defined, well-encapsulated and slightly lobulated margin. It was well demarcated from the surrounding structures and compressed the bladder anteriorly and rectum posteriorly. The precontrast image showed the mass had a homogenously lower attenuation than that of skeletal muscle. No calcification was noted in the mass (Fig. 1A). Arterial phase CT images after contrast administration showed that the mass was a heterogeneous enhancing lesion containing tubular enhancing structures, which were presumed to be blood vessels and multiple septa. Further, on 10 minute late CT images after contrast administration, the mass revealed strong enhancement (Fig. 1B, C). Magnetic resonance imaging (MRI) showed that the mass abutted to the bladder and rectum, and compressed the seminal vesicle inferiorly. Its signal intensity on both T1-weighted image (T1WI) and T2-weighted image (T2WI) was higher than that of skeletal muscles. Further, on the T2WI, the mass was found to be a tubular structure with a signal void and multiple intratumoral septa. After contrast administration, the mass showed heterogeneous enhancement with a poorly enhancing portion within it, similar to that seen on the CT scan (Fig. 1D-G). Hence, the mass was considered to be a soft tissue sarcoma arising in the extraperitoneal region.
At operation, the tumor was found to be located in the rectovesical space adhered to the bladder and the rectum. It was excised using mass excision, partial cystectomy, and cystoplasty.
On a histological examination, we found that the tumor was comprised of short-spindle cells with rich blood vessels and foci of geographic necrosis, and had myxoid changes. Moreover, the tumor cells were uniform and relatively small in size with inconspicuous nucleoli, contained sparse cytoplasm, and had distinct cell borders. Mitosis was occasionally observed. On immunohistochemical examination using formalin-fixed and paraffin-embedded tissue blocks, the tumor cells were found to be positive for vimentin, CD99, CD10 (focal), and Bcl-2 but negative for cytokeratin, EMA, CD34, CD56, CD117, desmin, smooth muscle actin, S100, and HMB45. On the basis of these histological and immunohistochemical features, the tumor was diagnosed as a monophasic synovial sarcoma showing myxoid change (Fig. 1H).
The rectovesical space is the region between the bladder and the rectum. It is surrounded superiorly by the peritoneum; inferiorly by the fascia overlying the posterior part of the urogenital diaphragm; laterally by the sheath arising from the parietal pelvic fascia that contains vessels, nerves, and lymphatics going to the prostate in the male and to the vagina, uterus, and ovaries in the female; and posteriorly by the 'rectal stalks'. This space is divided into the retrovesical and prerectal compartments by the rectovesical (Denovillier's) fascia in the male, and by the vagina and cervix uteri in the female (4). Thus far, only 2 reports have described primary tumors (lymphoma and dermoid cyst) arising in the rectovesical space (56).
Synovial sarcoma is an uncommon malignant mesenchymal tumor that accounts for 2.5-10.5% of all primary soft tissue malignancies worldwide (1). Although this tumor is seen across all age groups and at any anatomical location, most synovial sarcomas (80-95%) occur in the extremities, especially in the lower limbs around the knees (1, 3). Furthermore, in more than 90% of cases, synovial sarcomas are located in the para-articular regions in the limbs (1237). This tumor has been founded to arise in the extraperitoneal region, the most uncommon place of occurrence, occurring in only 0.3% of cases (12), for the most part arising in the abdominal retroperitoneum (38). Less than 10 cases of synovial sarcomas in the pelvic extraperitoneal region have been reported thus far, and these reports focus only on the pathologic findings (3). To the best of our knowledge, this is the first report on a synovial sarcoma arising in the rectovesical space.
Synovial sarcomas, irrespective of their location, have no specific imaging features, and this makes them indistinguishable from other mesenchymal tumors (179). The most common finding on CT is the heterogeneous attenuation of the mass, which is usually similar to or slightly lower than that of skeletal muscles (1). In addition, areas of low attenuation representing necrosis or hemorrhage are common, although smaller lesions may be mostly homogeneous (12). CT is a useful imaging technique for identifying calcifications, especially those that are subtle or located in regions with a complex anatomy (12). After administration of intravenous contrast agents, synovial sarcomas may show irregular peripheral enhancement; poor central area enhancement, reflecting necrotic, cystic, and hemorrhagic regions; and nodular enhancement (1). In our case, CT scans showed heterogeneous attenuation that was slightly lower than that of skeletal muscle on the precontrast image and poorly enhancing portions within the tumor and heterogeneous delayed enhancement on the postcontrast image. These features are similar to those of synovial sarcomas arising in other locations. However, it did not show calcification. The enhancement pattern of synovial sarcoma has not been reported to date. In this case, delayed enhancement of the mass was seen. We assumed this finding was caused by increased vascularity, cellularity, and myxoid change such as focal nodular hyperplasia of the liver. Further, a serpentine vascular channel was observed in the contrast-enhanced CT (Fig. 1B). This finding has not been reported thus far on CT, although Murphey et al. (1) identified the vascular channel on MRI. They reported the presence of the serpentine vascular channel in approximately one-third of synovial sarcomas, and stated that the use of these radiologic findings could limit the differential diagnoses to alveolar soft tissue sarcoma, metastatic renal cell carcinoma, hemangio-pericytoma, hemangioendothelioma, rhabdomyosarcoma, extraskeletal Ewing's sarcoma, and synovial sarcoma. Nishimura et al. (10) found that the flow void on MRI was common in the case of hemangiopericytoma, arteriovenous hemangioma, and alveolar soft part sarcoma.
Previous reports on the MRI findings of synovial sarcoma mentioned the triple sign, bowl of grapes appearance, intervening septa, neurovascular encasement, and calcification, but no serpentine vascular channel (179). In contrast, the synovial sarcoma in our case showed a vascular channel, triple sign, and intervening septa, but no neurovascular encasement, calcification, or bowl of grapes appearance. The combination of a large cystic area and hemorrhagic foci often leads to the latter feature (1). Jones et al. (9) reported marked signal heterogeneity of the mass on the T2WI as the triple sign. Pathologically, this finding reflects a mixture of solid elements (intermediate signal intensity), hemorrhagic or necrotic elements (high signal intensity), and calcified or fibrous elements (low signal intensity) (19). However, the triple sign is also observed in other soft tissue neoplasms, particularly malignant fibrous histiocytoma; therefore, this finding by itself is not specific (1). Moreover, T2WIs show the intervening septum in approximately 60% of synovial sarcomas (18), and this was also observed in our case.
In conclusion, the radiological findings of synovial sarcoma arising in the rectovesical space are similar to those of tumors at other locations. Synovial sarcomas in the rectovesical space are difficult to distinguish from other retroperitoneal soft tissue masses. However, if a soft tissue mass in the rectovesical space has intervening septa, vascular channels, and signal heterogeneity, synovial sarcoma should be included in the differential diagnosis.
Figures and Tables
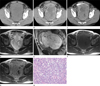
Fig. 1
The synovial sarcoma in the rectovesical space of a 48-year-old man.
A. Precontrast-phase CT scan shows a well-defined homogenous soft tissue mass without any calcification in the entire pelvic cavity.
B. Arterial phase CT scan shows an intratumoral tubular structure (arrows) indicating an enhanced vascular structure. Multiple enhancing septa (arrowheads) and poorly enhanced lesions are also seen in the mass.
C. 10 minute late CT scan after IV contrast administration shows strong enhancement in the mass.
D. T2-weighted axial MR image shows signal heterogeneity and multiple septa in the mass. An intratumoral serpentine tubular structure with a signal void (arrows) is seen.
E. T2-weighted sagittal MR image shows the mass surrounded anteriorly by the urinary bladder (white arrows) and posteriorly by the rectosigmoid colon (black arrows).
F. Precontrast T1-weighted axial MR image shows that the mass has a slightly higher signal intensity than that of the skeletal muscle.
G. Postcontrast T1-weighted axial MR image shows that the mass contains a strongly enhancing solid portion and poorly enhancing portion.
H. The tumor has uniform and relatively small ovoid or short-spindle cells with occasional mitosis (Hematoxylin-eosin staining, × 400).
References
1. Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. From the archives of the AFIP: Imaging of synovial sarcoma with radiologicpathologic correlation. Radiographics. 2006; 26:1543–1565.
2. Ulusan S, Kizilkilic O, Yildirim T, Hurcan C, Bal N, Nursal TZ. Radiological findings of primary retroperitoneal synovial sarcoma. Br J Radiol. 2005; 78:166–169.
3. Chatzipantelis P, Kafiri G. Retroperitoneal synovial sarcoma: a clinicopathological study of 6 cases. J BUON. 2008; 13:211–216.
4. Janke WH, Block MA. Chronic retroperitoneal pelvic abscesses. Arch Surg. 1965; 90:380–384.
5. Tosaka A, Yamazaki A, Hirokawa M, Matsushita K, Asakura S. [A bulky mass non-Hodgkin's lymphoma with dysuria in the rectovesical space]. Hinyokika Kiyo. 1990; 36:701–705.
6. Wilson RG. Dermoid cyst of the rectovesical space: report of a case. Dis Colon Rectum. 1973; 16:530–531.
7. Morton MJ, Berquist TH, McLeod RA, Unni KK, Sim FH. MR imaging of synovial sarcoma. AJR Am J Roentgenol. 1991; 156:337–340.
8. Tateishi U, Hasegawa T, Beppu Y, Satake M, Moriyama N. Synovial sarcoma of the soft tissues: prognostic significance of imaging features. J Comput Assist Tomogr. 2004; 28:140–148.
9. Jones BC, Sundaram M, Kransdorf MJ. Synovial sarcoma: MR imaging findings in 34 patients. AJR Am J Roentgenol. 1993; 161:827–830.
10. Nishimura H, Zhang Y, Ohkuma K, Uchida M, Hayabuchi N, Sun S. MR imaging of soft-tissue masses of the extraperitoneal spaces. Radiographics. 2001; 21:1141–1154.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download