Abstract
It has been well known that angiosarcoma (AS), particularly scalp AS, metastasizes to the lungs with multiple air-filled cystic lesions on chest computed tomography scans. Pneumothorax, due to cystic lesion rupture into the pleural space, is frequent; however, we do not exactly know how rapidly the metastatic lesions spread to the lungs or what the exact pathogenetic mechanism for cystic metastasis is. According to our experience, the speed of disease progression in pulmonary metastasis is relatively fast and the entire lungs may be involved within two or three months. The infiltrating spindle cell tumors in the alveolar walls are tethering the adjacent alveolar spaces in order to form a dilated air-filled cystic lesion.
Angiosarcoma (AS) is a rare malignant tumor that originates from endothelial cells, and has an extremely poor prognosis. AS is frequently accompanied by pulmonary metastasis with a presentation of thin-walled cavitary lesions. We report a patient with AS manifesting as spontaneous pneumothorax secondary to pulmonary metastasis, which increased in size over the clinical course of several months. This case illustrates the need for awareness of the aggressive nature of this tumor and to suspect metastasis when pulmonary complications develop.
An 82-year-old man presented with cough and mild dyspnea. The patient had a history of scalp AS diagnosed two months prior. The AS was diagnosed when it manifested as a palpable soft-tissue mass on his left forehead. For staging workup, chest radiograph and neck CT scans were taken. The neck CT showed an enhancing mass in his scalp at the left forehead area (Fig. 1A). The neck CT, which distally covered the apical portion of both lungs, demonstrated a small air-filled cystic lung lesion (Fig. 1B) in the right upper lobe. A chest radiograph was found to be normal; consequently, at that time, the cystic lesion was disregarded. The patient underwent wide resection surgery for his scalp AS.
During his follow-up period, the patient felt mild dyspnea, and thus underwent a follow-up chest radiograph and CT scans. The chest radiograph showed pneumothorax in the right upper lung zone. CT scans obtained two months after the initial neck CT showed multiple, evenly thin-walled and air-filled cystic lesions in both lungs, along with right pneumothorax. The small cyst in the right upper lobe, which had been identified on neck CT scans, showed enlargement in its dimension (Fig. 1C). Additionally, multiple new cystic lesions were identified in the contralateral left lung. At the lower level, multiple cystic lesions were also noticed (Fig. 1D). A closed thoracostomy was performed to evacuate the pneumothorax.
For tissue confirmation of the cystic lesions, a surgical lung biopsy using video-assisted thoracoscopic surgery, was conducted from one of the cystic lesions in the right upper lobe. Histopathology revealed metastatic lung AS. On CT-pathologic correlation, the cystic wall was composed of alveolar wall structures containing infiltrating spindle cell tumors. Within the lumen of the cystic lesion, blood clots due to hemorrhage, could be identified. On high magnification view, the alveolar walls were replaced by spindle-shaped or oval tumor cells forming slit-like blood vessels harboring internal red blood cells (Fig. 1E, F). The tumor cells showed strongly positive staining for CD31 and CD34 (Fig. 1F).
ASs are rare but highly malignant tumors arising from the vascular endothelium. Approximately half of the cases involve the head and neck regions. They occur most frequently in the sixth and seventh decades of life, with a male predilection (1). ASs tend to recur locally and to metastasize despite aggressive therapy. Most ASs are metastasized at the time of diagnosis and have a poor prognosis; 34% overall 5-year survival rate due to frequent recurrence and early hematogenous metastases (2). The most common metastatic sites include the lung, liver, and lymph nodes (23).
Pulmonary metastasis of ASs commonly appears as extensive solid nodules; but, cystic or cavitary pulmonary lesions have also been reported. In one series of 24 patients with pulmonary metastasis of ASs described by Tateishi et al. (4), multiple thin-walled cystic lesions were reported in 21% of patients. In one previous case report, cavitary metastasis to the lung from a AS of the scalp showed round or bizarre shaped, distinct thin-walled cavities, mimicking cysts of Langerhans cell histiocytosis (5). In this case report, pulmonary metastasis of AS of the scalp appeared as an evenly round shaped with thin-walled cavities, measuring about 2-14 mm in size. There was no discernible nodular component in the walls of cavities.
As for the pathogenesis of cyst formation in pulmonary metastatic AS, several mechanisms have been suggested: 1) infiltration of tumor cells in the peribronchiolar regions serving as a check-valve mechanism for the formation of cystic lesions distal to the corresponding small airways (5),2) excavation of the whole or a portion of solid metastatic nodules, and 3) infiltration of malignant cells into the walls of pre-existing benign pulmonary cystic lesions (36). But, in our case, CT-pathology correlation helped us to suggest a new pathogenetic mechanism for the cyst formation. The alveolar walls are replaced by proliferating vascular (capillary) structures (spindle cell tumor cells) containing internal red blood cells. The infiltrating spindle cell tumors in the alveolar walls are tethering the adjacent alveolar spaces in order to form a dilated air-filled cystic lesion. Within the lumen of the cystic lesion, there may be blood clots due to hemorrhage.
Pulmonary complications including pneumothorax, pulmonary hemorrhage, atelectasis, and pneumonia can occur in patients with pulmonary metastasis of ASs. Kitagawa et al. (3) reported about 11% of 33 patients with AS of the scalp developed spontaneous pneumothorax. The mechanism of pneumothorax development in pulmonary metastasis in ASs could be the result of the rupture of peripheral malignant cysts into the pleural space (78). And, once the pneumothorax occurs, it is usually recurrent and intractable, resulting in an unfavorable outcome (9).
In our case, the speed of metastatic lesion progression was relatively fast. Within two months, few lung cystic lesions demonstrated an increase in size. Moreover, additional multiple air-filled cysts newly appeared within the same follow-up period of two months. The rapid progression of cystic lung lesions could also be identified in another case report (6).
There has been no consensus on how we treat patients with metastatic AS. Doxorubicin- and/or paclitaxel-based chemotherapy can be administered. However, prognosis is grave with a median disease-specific survival of approximately 10 months (10).
In conclusion, ASs, particularly scalp ASs, have cystic pulmonary metastatic lesions. Pneumothorax, probably due to the intrapleural rupture of these subpleural cysts, may be the initial presentation in this particular condition. The speed of disease progression in pulmonary metastasis is relatively fast and the entire lungs may be involved within two or three months. The infiltrating spindle cell tumors in the alveolar walls are tethering the adjacent alveolar spaces in order to form a dilated air-filled cystic lesion. The metastatic lesions may be treated with Doxorubicin- and/or paclitaxel-based chemotherapy, but prognosis is guarded.
Figures and Tables
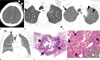
Fig. 1
Scalp angiosarcoma and cystic pulmonary metastasis in an 82-year-old man.
A. Enhanced brain CT (5-mm-section thickness) shows an enhancing scalp lesion (arrows) in the left forehead area.
B. Lung window image of thoracic CT (5.0-mm-section thickness) obtained at the same time to A demonstrates a 4-mm-sized air-filled cystic le-sion (arrow) in the right upper lobe.
C. Follow-up chest CT images (2.5-mm-section thickness) obtained at the same level to B, and two months after A and B, demonstrate the enlargement of a cystic lesion (arrow) in the right upper lobe along with additional multiple cystic lesions (arrowheads) in both lungs.
D. Coronal reformatted image (2.0-mm-section thickness) shows multiple cystic lesions in both lungs along with right pneumothorax (arrow).
E, F. Histopathologic and immunohistochemical staining for a surgical lung biopsy specimen.
E. Scanning view (H & E, × 1) of histopathologic specimen discloses a cystic lesion, the wall of which is composed of alveolar wall structures (ar-rows) containing infiltrating spindle cell tumors. The cyst has internal hemorrhage.
F. High-magnification view (H & E, × 200) reveals alveolar walls containing spindle-shaped or oval tumor cells forming slit-like vascular structures (arrows). The proliferated vasculature contains intraluminal red blood cells.
Inset. strongly positive for CD31 on immunohistochemical staining.
References
1. Mark RJ, Tran LM, Sercarz J, Fu YS, Calcaterra TC, Juillard GF. Angiosarcoma of the head and neck. The UCLA experience 1955 through 1990. Arch Otolaryngol Head Neck Surg. 1993; 119:973–997.
2. Morgan MB, Swann M, Somach S, Eng W, Smoller B. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004; 50:867–874.
3. Kitagawa M, Tanaka I, Takemura T, Matsubara O, Kasuga T. Angiosarcoma of the scalp: report of two cases with fatal pulmonary complications and a review of Japanese autopsy registry data. Virchows Arch A Pathol Anat Histopathol. 1987; 412:83–87.
4. Tateishi U, Hasegawa T, Kusumoto M, Yamazaki N, Iinuma G, Muramatsu Y, et al. Metastatic angiosarcoma of the lung: spectrum of CT findings. AJR Am J Roentgenol. 2003; 180:1671–1674.
5. Kim MY, Lim BS, Oh MH, Im JG. Metastatic angiosarcoma of the lung: HRCT findings. J Korean Radiol Soc. 1999; 40:493–496.
6. Park SI, Choi E, Lee HB, Rhee YK, Chung MJ, Lee YC. Spontaneous pneumomediastinum and hemopneumothoraces secondary to cystic lung metastasis. Respiration. 2003; 70:211–213.
7. Lawton PA, Knowles S, Karp SJ, Suvana SK, Spittle MF. Bilateral pneumothorax as a presenting feature of metastatic angiosarcoma of the scalp. Br J Radiol. 1990; 63:132–134.
8. Lee CH, Park KU, Nah DY, Won KS. Bilateral spontaneous pneumothorax during cytotoxic chemotherapy for angiosarcoma of the scalp: a case report. J Korean Med Sci. 2003; 18:277–280.
9. Goto H, Watanuki Y, Miyazawa N, Kudo M, Inoue S, Kobayashi N, et al. [Clinical and pathological analysis of 10 cases of secondary pneumothorax due to angiosarcoma of the scalp]. Nihon Kokyuki Gakkai Zasshi. 2008; 46:85–91.
10. Lahat G, Dhuka AR, Lahat S, Smith KD, Pollock RE, Hunt KK, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009; 16:2502–2509.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download