Abstract
Endovascular aneurysm repair (EVAR) has been accepted as an alternative to traditional open surgery in selected patients. Despite the minimally invasiveness of this treatment, several complications may occur during or after EVAR. Complications include endoleak, aortic dissection, distal embolism, or iatrogenic injury to the access artery. However, there are few reports on the vascular rupture caused by a molding balloon during EVAR. We report two cases of infrarenal abdominal aortic aneurysms complicated by procedure-related aortic or iliac artery rupture by the molding balloon during EVAR. In our cases, we observed suddenly abrupt increase of the diameter of the endograft during balloon inflation, because we inflated the balloon rapidly. In conclusion, careful attention must be paid during inflation of the molding balloon to prevent vascular rupture.
Since the report of the first endovascular repair of an abdominal aortic aneurysm (AAA) by Parodi et al. in 1991, endovascular aneurysm repair (EVAR) has been accepted as an alternative to traditional open surgery in selected patients (123). Despite the minimally invasiveness of this treatment, there are several complications that may occur during or after EVAR. Complications include endoleak, graft thrombosis, graft kinking and migration, aortic dissection, distal embolism, or iatrogenic injury to the access artery (rupture, dissection, and false aneurysm) (12345678).
A 65-year-old male with a history of percutaneous coronary intervention presented with a 5.4 cm infrarenal AAA. The computed tomographic angiography (CTA) revealed a 5.4 cm-diameter infrarenal AAA, without involving the iliac arteries. The AAA characteristics were in favor of using an endovascular graft. The patient was scheduled for EVAR.
Because the left common iliac artery (CIA) was too short (15 mm in length), the left internal iliac (IIA) was occluded with several metallic coils to prevent a type II endoleak. The main body of the aortic stent graft (Zenith, COOK, Bloomington, IN, USA) was deployed in the aorta through the femoral arteriotomy. Following deployment of the main body, the right limb was deployed in the right CIA and then left limb was deployed in the left external iliac artery (EIA) without any complication. To ensure complete graft expansion, the grafts were dilated with a molding balloon (Coda balloon catheter, COOK, Bloomington, IN, USA). During dilatation of the distal end of right iliac limb with balloon catheter, sudden expansion of the distal limb was felt and extravascular leakage of the contrast media on angiogram was found, but blood pressure and pulse rate were stable. For the treatment of unexpected right CIA rupture, occlusion of the right IIA with coils, followed by extension of an additional graft limb to the EIA were performed. The procedure was finished after confirming no more contrast leakage from the right CIA on a completion angiogram (Fig. 1).
After the procedure, transfusion with three units of RBC was required to control blood pressure and to correct the hemoglobin level at the recovery room. Under the apprehension of acute pelvic ischemia due to blood loss and simultaneous occlusion of both internal iliac arteries, a colonoscopy was performed at the third postoperative day, which revealed no evidence of colonic ischemia. CTA performed on the sixth postoperative day showed retroperitoneal hematoma without endoleak or contrast leakage from the right CIA (Fig. 1). The patient had an uneventful recovery and was discharged from the hospital on the seventh postoperative day from EVAR. A follow-up CTA at 6 months after EVAR showed a well-positioned and functioning endograft without any evidence of endoleak.
A 76-year-old male with a past history of radical subtotal gastrectomy for advanced gastric cancer presented with an enlarging infrarenal AAA. Preoperative CTA demonstrated a 5.9 cm sized infrarenal AAA with a 2.2 cm sized right CIA aneurysm.
Procedures were performed under general anesthesia with surgical arteriotomy of both femoral arteries. Before the main body deployment, embolization of the right IIA was performed to prevent endoleak from the IIA. The main body and both graft limbs were deployed via both the femoral arteriotomy sites and then the grafts were dilated with a molding balloon (Coda balloon catheter, COOK, Bloomington, IN, USA). An angiogram after balloon dilatation showed proximal type I endoleak. To treat proximal type I endoleak, a second ballooning was attempted with the same balloon catheter. Completion angiogram showed extravasation of contrast medium from the proximal neck of the AAA just below the left renal artery (Fig. 2). The patient developed hypotension (systolic blood pressure, 70 mmHg). For the control of aortic rupture, we reinserted the molding balloon and inflated it at the level of infrarenal aorta to seal the ruptured aorta, and the balloon was securely sutured at the right groin. After inflation of the balloon, an angiogram showed that renal blood flow was preserved and no leakage of contrast medium from the aorta was found through the pigtail catheter in the suprarenal aorta. The patient's blood pressure was normalized after inflation of the balloon. Emergency surgery was performed with occlusion of the aorta with balloon. At the operation field, we found a huge retroperitoneal hematoma around the AAA. An aneurysmal sac was opened under suprarenal aortic clamping and the fabric segment of the aortic stent graft close to the proximal end was transversely divided. After cutting individual suprarenal fixing wires with wire cutter, the wires were removed with great caution not to injure the aortic with the barbs. After the removal of the whole aortic stent graft, AAA repair was performed in the usual manner. The patient's postoperative course was uncomplicated and he was discharged from the hospital on the fifth postoperative day.
Several early complications of EVAR including endoleak, endograft limb obstruction, local vascular complications (groin hematoma, femoral artery injury, iliac artery rupture), and AAA rupture have been reported. Among them, the most common vascular complications during EVAR are iliac artery injury caused by high degree of iliac artery stenosis, tortuosity, and/or calcification (178). However, aortic or iliac artery rupture by a molding balloon dilatation as our cases is a rare complication. Thirteen Intra-operative aortic ruptures were identified from 270 EVAR patients. In four (1.4%, 4/270) of these patients, aortic rupture occurred from balloon dilatation of the proximal aortic neck. Aortic ruptures were managed by open repair, like our case 2 (9). Eleven intra-operative iliac ruptures were identified from 369 EVAR patients. Among them, one case of iliac rupture occurred after balloon dilatation of the iliac limb. This complication was managed by the extension of another iliac limb, as in our case 1 (8).
Because aortic rupture by molding balloon dilatation occurred at the proximal neck of the AAA, endovascular management by extension of another endograft was not possible and all reported cases were managed by open repair (9). In our case 2, because the ruptured site was just below the left renal artery, endovascular management was not considered as a good option. For the control of an aortic rupture, we immediately reinserted the molding balloon and inflated at the level of infrarenal aorta to seal off the ruptured aorta. No more extravasation of contrast medium and patent of both renal arteries were confirmed by angiography via 5 Fr. A pigtail catheter was inserted through the contralateral limb. After angiography, a pigtail catheter was removed and the molding balloon was securely sutured at the right groin to prevent migration. Emergency surgery was performed with occlusion of the aorta with a balloon catheter and the patient's postoperative course was uncomplicated. In patients with iliac artery rupture and hemodynamic compromise, the endovascular aortic occlusion balloon was introduced to stop the bleeding. After balloon occlusion, a limb of the endograft was deployed to repair the ruptured artery in most reported cases (8). In our case 1, for the treatment of unexpected right CIA rupture with stable vital sign, we performed occlusion of the right IIA with coils to prevent type II endoleak, followed by extension of additional graft limb to the EIA. We could finish the procedure after confirming no more contrast leakage from the right CIA on a completion angiogram.
Balloon catheters have two principal applications in peripheral endovascular procedures. Therapeutic or angioplasty ballooning is the forceful inflation of a balloon to dilate stenotic/occlusive lesions in a vessel. In contrast, molding or occlusion ballooning is a less forceful balloon inflation to ensure endograft expansion, placement, and fixation. This is done using larger compliant balloons and a more gentle inflation. These balloons are also used to stop blood flow in medium or large vessels as needed in certain vascular procedures. The law of Laplace states that the force or tension (T) exerted on the wall of the inflated balloon is directly proportional to the pressure (P) within the balloon and the radius of the balloon (T = P × R). Therefore, larger balloons will require less pressure than smaller balloons to generate a substantial dilating force. Similarly, large vessels such as the abdominal aorta or the common iliac arteries require less pressure to dilate and rupture. The Coda Balloon is very useful in endograft molding and aortic occlusion. According to the User's manual, this balloon is composed of a compliant polyurethane material, which facilitates the rapid inflation and deflation, which is advantageous when occluding the aorta. However, a manual recommended slow inflation of the balloon is recommended to prevent vascular rupture. In our cases, we observed a sudden abrupt increase of the diameter of the endograft during balloon inflation, because we inflated the balloon rapidly. Though there has been no detailed description of the cause of vascular rupture during balloon inflation, we think that gentle slow inflation of the balloon is important to prevent vascular rupture (91011).
In conclusion, full graft expansion with a molding balloon is of great importance during EVAR because full graft expansion will minimize the chance of endoleak, and residual graft stenosis is the primary etiologic factor of graft limb thrombosis. However, careful attention must be paid during inflation of the molding balloon to prevent vascular rupture as in our cases.
Figures and Tables
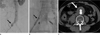 | Fig. 1A. Angiogram shows leakage of contrast medium due to the rupture of the right common iliac artery (black arrow).
B. Additional extension of the graft limb (black arrow) to the external iliac artery was performed to exclude rupture of the common iliac artery.
C. CTA performed on the sixth postoperative day shows the remaining hematoma in the retroperitoneum (white arrow) without showing an endoleak or active bleeding from the iliac artery.
Note.-CTA = Computed tomographic angiography
|
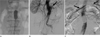 | Fig. 2A. Angiogram after balloon dilatation showed proximal type I endoleak. To treat proximal type I endoleak, a second ballooning was attempted.
B. Completion angiogram showed the extravasation of contrast medium from the proximal neck of the AAA (black arrows) just below the left renal artery.
C. After inflation of the balloon, renal blood flow is preserved (black arrows) and no leakage of contrast medium from the aorta was confirmed by angiogram through the pigtail catheter in the suprarenal aorta.
Note.-AAA = abdominal aortic aneurysm
|
References
1. Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004; 351:1607–1618.
2. Becquemin J, Bourriez A, D'Audiffret A, Zubilewicz T, Kobeiter H, Allaire E, et al. Mid-term results of endovascular versus open repair for abdominal aortic aneurysm in patients anatomically suitable for endovascular repair. Eur J Vasc Endovasc Surg. 2000; 19:656–661.
3. Nevala T, Biancari F, Manninen H, Aho PS, Matsi P, Mäkinen K, et al. Finnish multicenter study on the midterm results of use of the Zenith stent-graft in the treatment of an abdominal aortic aneurysm. J Vasc Interv Radiol. 2009; 20:448–454.
4. Mehta M, Sternbach Y, Taggert JB, Kreienberg PB, Roddy SP, Paty PS, et al. Long-term outcomes of secondary procedures after endovascular aneurysm repair. J Vasc Surg. 2010; 52:1442–1449.
5. Drury D, Michaels JA, Jones L, Ayiku L. Systematic review of recent evidence for the safety and efficacy of elective endovascular repair in the management of infrarenal abdominal aortic aneurysm. Br J Surg. 2005; 92:937–946.
6. Aljabri B, Obrand DI, Montreuil B, MacKenzie KS, Steinmetz OK. Early vascular complications after endovascular repair of aortoiliac aneurysms. Ann Vasc Surg. 2001; 15:608–614.
7. Liaw JV, Clark M, Gibbs R, Jenkins M, Cheshire N, Hamady M. Update: complications and management of infrarenal EVAR. Eur J Radiol. 2009; 71:541–551.
8. Fernandez JD, Craig JM, Garrett HE Jr, Burgar SR, Bush AJ. Endovascular management of iliac rupture during endovascular aneurysm repair. J Vasc Surg. 2009; 50:1293–1299.
9. Schlösser FJ, Gusberg RJ, Dardik A, Lin PH, Verhagen HJ, Moll FL, et al. Aneurysm rupture after EVAR: can the ultimate failure be predicted. Eur J Vasc Endovasc Surg. 2009; 37:15–22.
10. Becker GJ. Balloon angioplasty catheter. In : Baum S, Pentecost MJ, editors. Abrams' Angiography Interventional Radiology. Philadelphia: Lippincott Williams and Wilkins;2006. p. 187–204.
11. Sarin S, Turba UC, Angle F, Hagspiel KD, Dake MD, Matsumoto AH. Balloon catheter. In : Mauro MA, Murphy KP, Thomson KR, Venbrux AC, Zollikofer CL, editors. Image Guided Intervention. Philadelphia: ElsevierSaunders;2008. p. 75–84.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download