Abstract
We report here on the imaging findings of the case of KIT-negative gastrointestinal stromal tumor (GIST) in the stomach of a 12-year-old girl. Radiologic studies revealed the presence of a huge exophytic growing mass that originated from the gastric wall and this mass consisted of solid and cystic components on USG, CT and MR. The cystic regions were mainly located at the periphery of the mass and they were revealed to be myxoid degeneration and hemorrhage on histopathologic examination. The tumor consisted of epithelioid and typical spindle cells and they showed negative immunoreactivity for KIT. Although KIT-negative GISTs are rare, they can be considered in the differential diagnosis when a large heterogeneous extraluminal mass that contains solid portions and various degrees of peripheral cystic regions is observed.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumor among the gastrointestinal tumor in adults, yet these are uncommon tumors in pediatric patients. The clinical and histopathological features of GISTs in children differ from those in adults (12). GISTs are well known for their immunoreactivity for KIT (CD 117, a stem-cell factor receptor), but a few GISTs show a weak or negative KIT expression (345). Although the KIT-negative GISTs usually show the typical clinicopathologic features of the conventional KITpositive GISTs, they have some unique features in some aspects. We report here on the imaging findings of a KIT-negative gastric GIST in a 12-year-old girl.
A 12-year-old girl who presented with a palpable abdominal mass was referred to our institution. She had experienced abdominal pain for the previous few days and the physical examination revealed a huge mass in the epigastric area. All laboratory findings were within the normal limits (hemoglobin: 12.6 g/dL, hematocrit: 35.8%, RBC count: 4.20 × 103/µl, WBC count: 5.40 × 103/µl, platelet count: 221 × 103/µl). Abdominal ultrasonography (USG) revealed the presence of a huge mass that measured 14 × 11 cm, it was abutting the gastric wall and it showed mixed echogenicity in the cystic and solid regions (Fig. 1A). Computed tomography (CT) and magnetic resonance imaging (MRI) were performed to accurately determine the location of the mass and to characterize it. CT indicated that the mass was abutted to the wall of the lower gastric body and it had grown exophytically into the abdominal cavity. The mass consisted of cystic and solid regions, as observed on USG, and it showed heterogenous contrast enhancement (Figs. 1B-D). MRI also suggested that the mass surfaced from the gastric wall and it contained peripheral cystic regions that showed homogenous low signal intensity on the T1-weighted image and homogeneous high signal intensity on the T2-weighted image. The solid regions revealed intermediate signal intensity on both the T1- and T2-weighted images and slight contrast enhancement on the contrast-enhanced T1-weighted MR image (Figs. 1E-G). The findings also indicated that the mass had not infiltrated the surrounding structures or metastasized to the liver or lymph nodes. The radiologic diagnosis was a GIST arising from the gastric wall, although GISTs have been rarely reported in children. The mass was removed by gastric wedge resection. Examination of the gross specimen showed that the mass originated from the gastric submucosa, and myxoid degeneration with cystic changes and hemorrhage was noted (Fig. 1H). These findings correlated with the findings of USG, CT and MRI.
Microscopically, the tumor was composed of 2 types of cells: the epithelioid cells were present in the myxoid region and there were also spindle cells. Although the tumor was negative for a KIT expression, the diagnosis of GIST was made on the basis of the fact that the tumor showed the typical clinicopathologic features of GISTs. On the immunohistochemical analysis, the tumor showed positive immunoreactivity for CD 34. Analyses for the expression of other cell markers such as desmin, neuron-specific enolase (NSE), S-100 protein and smooth muscle actin (SMA) were all negative.
GISTs rarely occur in children and the clinicopathologic features of GISTs in children differ from those in adults (12). A review of the articles on GISTs in children and young adults suggests that pediatric GISTs tend to frequently occur in girls and they predominately affect the stomach (1). In adults, there is no difference of the incidence of GISTs between men and women and the stomach is the most common site (6). The common clinical symptoms of pediatric GISTs are chronic anemia, a palpable mass and abdominal pain, which occur in 86.4%, 11.9% and 15.3% of the patients, respectively (2).
Histopathologically, most GISTs that occur in adults typically consist of spindle cells (6); however, pediatric GISTs predominantly show an epithelioid morphology. The other characteristics of pediatric GISTs include the frequent involvement of lymph nodes and the lack of a KIT expression or platelet-derived growth factor receptor (PDGFR) mutations (1). In this case, the tumor affected the stomach of a 12-year-old girl and the tumor had mixed cell types, including epithelioid cells and the typical spindle cells. However, no predominant cell type was observed and the lymph nodes were not involved.
Most GISTs express CD 117 (95%), CD 34 (70%) and heavy caldesmon (80%), whereas 25% are positive for a SMA expression and less than 5% are positive for a desmin expression, but a few tumors show a weak or negative KIT expression even though they show the typical clinicopathologic and cytogenetic features of GISTs (34567). CD 34 antigen is not specific for GISTs, but it is commonly present in GISTs (6). KIT-negative GISTs predominantly occur in the stomach, omentum or mesentery and they usually show an epithelioid or mixed epithelioid-spindle cell morphology on histopathologic examination (345). The diagnosis of GIST was made in this case, although the tumor showed no immunoreactivity for KIT (CD117), because the tumor consisted of epithelioid cells and the typical spindle cells and the tumor was positive for a CD 34 expression, but it was negative for SMA and desmin expressions. Other markers or a cytogenetic analysis can be used for making the diagnosis of GIST in the absence of KIT immunoreactivity. Particularly, the presence of KIT/PDGFR mutation is almost pathognomonic for the diagnosis of GIST. However, regardless of KIT positivity, GISTs do not always contain the KIT/PDGFR mutation (357). Unfortunately, cytogenetic study was not performed in this case.
Sakurai et al analyzed 30 GISTs that were weakly positive or totally negative for KIT. They introduced the concept of "myxoid epithelioid GISTs", which contained myxoid stroma with less cohesive epithelioid cells (7). In our case, the tumor consisted of 2 types of cells: epithelioid cells in a myxoid background and spindle cells. Interestingly, these features are very similar to those of pediatric KIT-positive GISTs.
Tateishi et al reported on 10 GISTs that were weakly positive or totally negative for KIT. According to their study, CT and MR indicated the presence of a large heterogeneous mass that contained cystic regions and various degrees of soft-tissue elements. Pathologically, all the tumors were soft-tissue masses with cystic regions. Myxoid degeneration was frequently present in the cystic regions, and necrosis and hemorrhage were present in a few lesions (4). However the cystic regions of the conventional KIT-positive GISTs were usually necrotic or apoptosis was present (89). In our case, the tumor also contained many cystic regions with soft-tissue elements. On the histopathologic examination, myxoid degeneration and hemorrhage were noted in the cystic regions. Unlike the KIT-positive GISTs that have shown central necrosis (8), the cystic regions in this case were present on the periphery of the tumor. A previous article presented the imaging findings of 3 cases of KIT-negative GISTs, and in 2 of these cases, the cystic regions were present on the periphery of the tumor (4). Thus, the cystic changes caused by myxoid degeneration in KIT-negative GISTs seemed to be present more peripherally than those in the KIT-positive GISTs. However, we could not find any reference showing whether peripheral myxoid degeneration was commonly present in the KIT-negative GISTs or in other tumors, except for neurogenic tumors. Peripheral myxoid degeneration is typically present in some schwannomas (10).
The differential diagnosis of gastric GIST that occurs in childhood consists of lymphoma, neuroendocrine tumor, neurogenic tumor, etc. Lymphoma usually tend to be solid tumor without a cystic portion, but neurogenic tumors and neuroendocrine tumors can contain cystic portions, so making the radiologic differentiation of these tumors is difficult.
It is very interesting that our case showed the typical clinical, radiologic and pathologic characteristics of both pediatric and KIT-negative GISTs. To the best of our knowledge, this is the first case report of a pediatric KIT-negative GIST in the medical literature. Although the occurrence of pediatric GISTs or KIT-negative GISTs is rare, the thorough knowledge of the clinical, radiological and pathological features of pediatric and KIT-negative GISTs will be helpful for making the differential diagnosis. In conclusion, KIT-negative GISTs can be considered when a large heterogeneous extraluminal mass with peripheral cystic regions and various degrees of soft-tissue elements is observed.
Figures and Tables
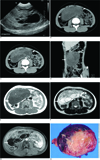 | Fig. 1A 12-year-old girl presented with a KIT-negative gastrointestinal stromal tumor of the stomach.A. Abdominal ultrasonography shows a huge mass with heterogenous echogenicity and the mass is composed of cystic and solid regions.
B, C. Axial pre- and post- contrast CT images show a huge mass (arrows) occupying the abdominal cavity. The mass shows enhancing solid and non-enhancing cystic components.
D. Coronal contrast-enhanced CT image shows a heterogeneously enhanced exophytic mass (arrow) originating from the gastric wall of the lower body (asterisk).
E-G. Axial T1-weighted MR image (E), T2-weighted MR image (F) and contrast-enhanced T1-weighted MR image (G) show a complex mass with cystic and solid regions. The solid regions (asterisk) have intermediate signal intensity on both the T1- and T2-weighted images with slight contrast enhancement on the contrast-enhanced T1-weighted image. The cystic regions (arrow) show low signal intensity on the T1-weighted image and high signal intensity on the T2-weighted image without contrast enhancement on the contrast-enhanced T1-weighted image.
H. Photograph of gross pathologic specimen revealed that the tumor had cystic regions that showed myxoid degeneration (arrow) and hemorrhage (asterisk).
|
References
1. Prakash S, Sarran L, Socci N, DeMatteo RP, Eisenstat J, Greco AM, et al. Gastrointestinal stromal tumors in children and young adults: a clinicopathologic, molecular, and genomic study of 15 cases and review of the literature. J Pediatr Hematol Oncol. 2005; 27:179–187.
2. Kaemmer DA, Otto J, Lassay L, Steinau G, Klink C, Junge K, et al. The Gist of literature on pediatric GIST review of clinical presentation. J Pediatr Hematol Oncol. 2009; 31:108–112.
3. Debiec-Rychter M, Wasag B, Stul M, De Wever I, Van Oosterom A, Hagemeijer A, et al. Gastrointestinal stromal tumours (GISTs) negative for KIT (CD117 antigen) immunoreactivity. J Pathol. 2004; 202:430–438.
4. Tateishi U, Miyake M, Maeda T, Arai Y, Seki K, Hasegawa T. CT and MRI findings in KIT-weak or KIT-negative atypical gastrointestinal stromal tumors. Eur Radiol. 2006; 16:1537–1543.
5. Medeiros F, Corless CL, Duensing A, Hornick JL, Oliveira AM, Heinrich MC, et al. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol. 2004; 28:889–894.
6. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review of morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006; 130:1466–1478.
7. Sakurai S, Hasegawa T, Sakuma Y, Takazawa Y, Motegi A, Nakajima T, et al. Myxoid epithelioid gastrointestinal stromal tumor (GIST) with mast cell infiltrations: a subtype of GIST with mutations of platelet-derived growth factor receptor alpha gene. Hum Pathol. 2004; 35:1223–1230.
8. Sandrasegaran K, Rajesh A, Rushing DA, Rydberg J, Akisik FM, Henley JD. Gastrointenstinal stromal tumors: CT and MRI findings. Eur Radiol. 2005; 15:1407–1414.
9. Kim HC, Lee JM, Kim SH, Park SH, Lee JW, Lee M, et al. Small gastrointestinal stromal tumours with focal areas of low attenuation on CT: pathological correlation. Clin Radiol. 2005; 60:384–388.
10. Suh JS, Abenoza P, Galloway HR, Everson LI, Griffiths HJ. Peripheral (extracranial) nerve tumors: correlation of MR imaging and histologic findings. Radiology. 1992; 183:341–346.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download