Abstract
Purpose
To analyze chest radiographic findings in children infected with laboratory-confirmed novel swine-origin influenza A (H1N1) virus.
Materials and Methods
Three hundred seventy-two out of 2,014 children with laboratory confirmed H1N1 infection and who also underwent a chest radiograph from September to November 2009 were enrolled in this study. Patients were divided into in-patients, out-patients, and patients with co-infections and further subdivided into with underlying disease and without underlying disease as well as age (<2 years old, 2-5 years, 5-10 years, 10-18 years old). The initial radiographs were evaluated for radiographic findings and the anatomic distribution of abnormalities.
Results
The initial radiographs were abnormal in 154 (41.39%) patients. The predominant radiographic findings were peribronchial wall opacity found in 85 (22.84%) patients and hyperinflation observed in 69 (18.54%) patients. Further, 75 (71.42%) patients exhibited central predominance and the right lower lung zone was also commonly involved. There were statistically significant differences in the radiological findings between in-patient and out-patient groups. However, there were no significant differences in the radiographic findings between in-patients and the co-infection group with respect the presence of underlying disease and age.
The first isolation of the swine influenza virus from a human occurred in 1974 (1), confirming the speculation that swine-origin influenza viruses could infect humans. Since 1975, sporadic human infections with swine viruses and a small outbreak have been documented (23456789). The swine flu outbreak that occurred in Mexico in April 2009 (10) rapidly spread to many countries around the world. In June 2009, the World Health Organization (WHO) declared the emergence of a global pandemic, raising the alert level to phase 6 (pandemic phase), on the basis of documented human-to-human spread of infection in at least three countries from two WHO regions. In Korea, there have been 252 deaths through April, 2010 and among them, 54% of the patients have been younger than 19 years of age (www.cdc.go.kr).
Normal or prominent peribronchial opacities with hyperinflation have been reported as common findings in children, whereas air space consolidation has been the most common finding in adults (111213). To date, the description of the radiologic manifestations of S-OIV has been limited in children. Therefore, the aim of this study was to analyze chest radiographic findings in children infected with laboratory-confirmed novel swine-origin influenza A (H1N1) virus (S-OIV).
This study was approved by the institutional review board with a waiver of informed consent. The study population included all patients less than 18 years of age with laboratory confirmed H1N1 from September 2009 to November 2009. Respiratory specimens were analyzed by real-time reverse transcription polymerase chain reaction (RT-PCR) (Artus Infl./H1LC/RG RT-PCR kit, Qiagen, Hilden, Germany). A total of 2014 patients were identified, among whom, 375 underwent chest radiographs. The indications for chest radiographs were respiratory symptoms with high fever (>38℃), abnormal breathing sound on auscultation, or those requested by parents. Other than those with urinary tract infection (n = 2) and poor image quality (n = 1), a total of 372 patients were enrolled in the study.
The common presenting symptoms of total laboratory confirmed H1N1 patients (n=2014) were fever (95.48%), cough (65.30%), and sore throat (20.41%). Further, 2.18% of patients had abdominal pain, 1.69% vomiting, and 0.55% had diarrhea.
On the initial chest radiographic images, lung parenchyma was classified as normal or abnormal. The initial radiographs were evaluated for parenchymal lesions (peribronchial wall opacity, hyperinflation, air space consolidation, atelectasis, or pneumomediastinum), anatomic distribution, (unilateral/bilateral, central/peripheral, diffuse, or upper/middle/lower lung zone), pleural effusion, and lymphadenopathy.
Radiologic findings were classified as peribronchial wall opacities with increased peribronchial cuffing with/without some nodularity. Hyperinflation was evident on the chest radiographs in children with hyperlucency, depression and/or flattening of the diaphragms to more than 10 posterior ribs, increased anterior to posterior chest diameter, and an increased retrosternal space. Air space consolidation referred to a density conforming to the lobar or segmental boundaries with evidence of volume expansion or minimal volume loss of the involved lobe and fluffy or homogeneously increased opacities with air- bronchograms. Atelectasis referred to densities conforming to lobar or segmental boundaries having evidence of volume loss. Pneumomediastinum was defined as the presence of one or more gas shadows within the mediastinum that did not correspond in position and contour with gas in the esophagus or tracheobronchial position. Pleural effusion was present under conditions of costophrenic angle blunting or fluid shifting on the decubitus view. Lymphadenopathy referred to lumpy round increased opacities at the outer edge of the hilum. In cases with peribronchial opacities, a quantitative estimate was recorded as absent, mild, moderate, or severe, and it was regarded as a positive finding when occurring at a moderate degree. The anatomic distribution was characterized as unilateral or bilateral, with each lung divided into upper, middle, and lower lung zones. The pattern was classified as predominantly central, peripheral, or diffuse. Further, the number of days of hospitalization was assessed in patients with extensive disease involving three lung zones.
The 372 H1N1-infected patients were divided into three categories: in-patients (n=73), out-patients (n=286) who had H1N1 infection only and those patients with co-infections (n=13); and further subdivided into with or without underlying disease and according to age (< 2 years old, 2 - 5 years, 5 - 10 years, and 10 - 18 years). We then evaluated the differences in radiologic findings for each of the groups. Also we evaluated differences with respect to hospitalization in the in-patient and co-infection groups. The in-patient group included patients who were initially admitted to the emergency room or visited the out-patient clinic and were later admitted. Patients with co-infection included 11 with Mycoplasma pneumoniae, 1 with rhinovirus and adenovirus, and 1 with Epstein-Barr virus.
Eighty-six patients (25.09%) had chronic medical conditions including: asthma (n = 73), preterm delivery with low birth weight (n = 12), a medicated seizure disorder (n = 2), Wilson's disease receiving penicilamine treatment (n = 1), tuberculosis (n = 1), and nephrotic syndrome (n = 1). Eighty-four of the 86 patients with chronic medical conditions were obtained with chest radiographs (16 in-patients, 65 out-patients, and 3 co-infected patients).
The posteroanterior/anteroposterior (PA/AP) and lateral projection radiographs were performed with digital radiography equipment (Gold mountain medical, TDR4600-F80, Seoul, Korea). The images were reviewed on a PACS viewer with a 2,560 × 2,048 pixel monitor (INFINITT, STAR PACS, v. 5081, Seoul, Korea). The chest radiographs were independently analyzed by two pediatric radiologists (HS Hong, YW Chang) at different times, and the differences were then evaluated by consensus. The K-value was used for the inter-observer agreement rate. As such, K-values < 0 indicated that inter-observer agreement was less than the agreement by chance alone. K-values between 0.01-0.20 indicated that, inter-observer agreement was slight: 0.21-0.40 indicated fair agreement, 0.41-0.60 indicated moderate agreement, 0.61-0.80 indicated good agreement, and 0.81-0.99 indicated excellent agreement. The differences in chest radiographic abnormalities and anatomic distribution with respect to underlying diseases were analyzed using the Fisher's exact test for categorical data and the Mann-Whitney test for continuous data. Statistical analyses were performed with the STATA computer software (Stata, College station, TX., USA). A p-value < 0.05 was the threshold for statistical significance.
Initial radiographs were abnormal in 154 of 372 (41.39%) patients. The anatomical distribution was characterized as being unilateral (71/105, 67.62%), or with bilateral involvement (34/105, 32.38%). The predominant radiographic findings were peribronchial wall opacity (85/372; 22.84%) (Fig. 1), and hyperinflation (69/372; 18.54%). Moreover, the most common pattern was central (75/105; 71.42%), which also affected the right lower lung zone (71/105; 67.62%). Air space consolidation was observed in 30 patients (30/372; 8.06%) (Fig. 2), while pleural effusion was observed in 11 patients (11/372; 2.95%). None of the patients showed lymph node enlargement. Extensive disease involving three lung zones was observed in 44/372 (11.83%) patients, resulting in a longer hospitalization time (mean: 5.54 days). However, the difference was not statistically significant (p > 0.05, Mann-Whitney test). The imaging findings have been summarized in Table 1. Inter- observer agreement with regard to the radiologic findings was good to excellent.
The first available chest radiographs were abnormal in 54/73 (73.97%) patients that had been admitted to hospital, 90/286 (31.47%) patients from the out-patient group, and 10/13 (76.92%) of the patients with co-infection (Table 2). Among the in-patients with abnormal radiographs, the predominant radiographic findings were peribronchial opacity (35/73, 47.94%), hyperinflation (21/73, 28.77%), and consolidation (19/73, 26.03%) in the in-patient group, while the common findings in the out-patient group were normal (196/286, 68.53%), with peribronchial wall opacities (45/286, 15.73%) and hyperinflation (46/286, 16.08%) (Table 2). Each of the differences in the imaging findings were statistically significant (p < 0.05), except for lymphadenopathy (Table 2). For patients with co-infection, the predominant chest radiographic finding was air space consolidation (6/13, 46.15%). Further, the percent of cases with pleural effusion (3/13, 23.08%) was higher than in the in-patient group (8/73, 10.96%); however, there were no statistically significant differences in the radiographic findings between the in-patient group and the patient with co-infections (p > 0.05) (Table 2). The median hospitalization duration for the in-patient group was 5.19 days (range 3~10 day), with a 6.00-day median (range 4~9 day) for patients with co-infections; this difference was not statistically significant. There were no differences in the radiographic findings between the groups with or without underlying disease (p > 0.05). In addition, there were no statistically significant differences in the radiographic findings according to age. The most common radiographic finding, with the exception of a normal result, was peribronchial opacities in the under 2 years of age group (2.69%); peribronchial opacity and hyperinflation were common in the 2-18 years group. There were no parenchymal or pleural complications. All patients improved with regard to clinical symptoms and chest radiographic findings. The number of days to an improved chest radiograph was a median of 5.43 days (range, 2-17 days).
Influenza A viruses are ubiquitous in nature. Pigs have been known to be infected with both avian and human influenza viruses, enabling these viruses to interact and generate new forms (14). A new strain of triple-reassortant influenza A (H1N1) virus containing genes from swine, avian and human influenza viruses was identified in Mexico in April 2009, and rapidly spread worldwide (10). Avian-like swine influenza A (H1N1) viruses can infect humans directly, without the need for a reassortment event; resulting in a serious disease threat. Some of the characteristics of H1N1 viruses differ from the seasonal influenza virus, such as their affects on younger patients. The clinical outcomes of this pathogen have been reported as ranging from self-limited illness to respiratory failure and death (1015). In the current study, most patients presented with flu- like symptoms such as fever, cough, sore throat, and sputum production. Abdominal symptoms were not common (4%) compared to other reports (25%) (15). The current study showed that about 60% of laboratory confirmed patients had normal radiographs. Among those with radiographic abnormalities, the most common finding was peribronchial increased opacities, similar to other reports of H1N1 in children (13) and other viral pneumonia in children (141617181920). Parahilar peribronchial infiltrates, hyperexpansion, segmental or lobar atelectasis, and hilar adenopathy have been common radiographic findings in the viral respiratory tract infections of children. The results of this study confirmed these findings (13161718192021).
Agarwal et al. (11) concluded that the most common abnormality in H1N1 adults was air-space disease (50%), with a predilection for lower and central lung preponderance in hospitalized patients requiring advanced mechanical ventilation. In this study, anatomic distribution or central predominance was consistent with prior reports; however, the frequency of air space consolidation was lower. Lee et al. (13) concluded that patients with a mild and self- limited clinical course had normal radiographs or prominent peribronchial markings with hyperinflation; a finding which was supported by this study. However, air space consolidation occurred with a high frequency in their study; with 57% (12/21) of hospitalized children, and 86% (12/14) of children who required intensive care treatment.
In contrast a report by Lee et al. (13), this study showed no differences in the radiographic findings between patients admitted to the hospital and those with co-infections (Table 2). This discrepancy was likely due to the different study populations.
Again, in contrast to the report by Lee et al. (13), there were no differences in the radiographic findings between a group with underlying disease and without underlying disease. It was presumed that patients with underlying disease would have had a more severe pattern of disease; however, there was no difference in radiographic findings among the groups. This may be explained by the fact that the most common underlying disease was asthma, and that there were few additional risk factors in this study.
Mycoplasma pneumonia was the most common organism affecting patients with co-infection, accountings for up to 30% of cases with pneumonia in school-aged children (22). The presence of upper respiratory tract viral infections is often complicated by more serious bacterial diseases. The influenza virus alters the host in a way that predisposes one to adherence, invasion, and induction of disease by pneumococcus. As such, Group A streptococcus, Staphylococcus aureus, and Streptococcus pneumoniae have been found to be common offending organisms (23).
Children under the age of 5 (15) or 2 years of age (17) have been considered high risk groups. However, in this study, there were no differences in the duration of chest radiographic findings or hospital stays according to age. Agarwal et al. (11) reported that patients with severe disease were also at risk for developing a pulmonary embolism: a high frequency of obesity was also noted as a significant patient factor in prior studies. In this study, there were no patients that required mechanical ventilation, that were obese, or that of a developed a pulmonary embolism. In this study, there were also no patients who needed admission to intensive care, or mechanical ventilation and there were no deaths. These findings were probably due to the public and physician awareness of the H1N1 virus in the community), which led to prompt diagnosis and treatment. This result reflected the lower mortality rate of H1N1 (0.017%) in Korea than seasonal influenza virus (0.1%) (www.cdc.go.kr) as well as a lower percentage observed in the USA (0.021%) (www.cdc.gov).
The limitations of this study stemmed from the fact that there were no patients with severe illness who also needed mechanical ventilation or who had developed respiratory failure. Likely, this was likely due to early recognition, diagnosis, and treatment. In fact, only a limited number of patients had risk factors such as asthma or, prematurity.
In conclusion, the initial radiographs were abnormal in 41.39% patients with H1N1 infection. Peribronchial wall opacities, hyperinflation, lower lung zonal distribution, and central predominance were the common radiographic findings among children infected with H1N1 virus.
Figures and Tables
Fig. 1
A 10-year-old boy with laboratory confirmed S-OIV (H1N1) who presented with coughing.
Chest PA (A), right lateral view (B) on hospital admission showed peribronchial opacities in the RLL and RML. The distribution showed a central predominance as well as a presence in the lower and middle lung zone.
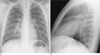
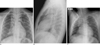
Fig. 2
A 7-year-old boy who presented with dyspnea and had a history of allergic rhinitis.
Chest PA (A), right lateral (B) and right decubitus view (C) on hospital admission showed consolidation with air-bronchogram in the BLL, RUL and increased peribronchial opacities in the right perihilar region, pneumomediastinum, subcutaneous emphysema, as well as fluid shifting in the dependent portion. The distribution of the lesion showed central predominance as well as diffuse involvement of the right lung.
References
1. Smith TF, Burgert EO Jr, Dowdle WR, Noble GR, Campbell RJ, Van Scoy RE. Isolation of swine influenza virus from autopsy lung tissue of man. N Engl J Med. 1976; 294:708–710.
2. Ito T, Couceiro JN, Kelm S, Baum LG, Krauss S, Castrucci MR, et al. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol. 1998; 72:7367–7373.
3. De Jong JC, Paccaud MF, de Ronde-Verloop FM, Huffels NH, Verwei C, Weijers FF, et al. Isolation of swine-like influenza A (H1N1) viruses from man in switzerland and the netherlands. Ann Inst Pasteur Virol. 1988; 139:429–437.
4. Goldfield M, Bartley JD, Pizutti W, Black HC, Altman R, Halperin WE. Influenza in New Jersey in 1976: isolations of influenza A/New Jersey/76 virus at Fort Dix. J Infect Dis. 1977; 136:Suppl. S347–S355.
5. Rota PA, Rocha EP, Harmon MW, Hinshaw VS, Sheerar MG, Kawaoka Y, et al. Laboratory characterization of a swine influenza virus isolated from a fatal case of human influenza. J Clin Microbiol. 1989; 27:1413–1416.
6. Wells DL, Hopfensperger DJ, Arden NH. Swine influenza virus infections: transmission from ill pigs to humans at a wisconsin agricultural fair and subsequent probable person-person transmission. JAMA. 1991; 265:478–481.
7. Wentworth DE, Thompson BL, Xu X, Regnery HL, Cooley AJ, McGregor MW, et al. An influenza A (H1N1) virus, closely related to swine influenza virus, responsible for a fatal case of human influenza. J Virol. 1994; 68:2051–2058.
8. Gregory V, Bennett1 M, Thomas Y, Kaiser L, Wunderli W, Matter H, et al. Human infection by a swine influenza A (H1N1) virus in Switzerland Brief Report. Arch Virol. 2003; 148:793–802.
9. Top FH, Russell PK. Swine influenza at Fort Dix, New Jersey (January-Febuary 1976). IV. Summary and speculation. J Infect Dis. 1977; 136:Suppl. S376–S380.
10. Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009; 361:680–689.
11. Agarwal PP, Cinti S, Kazerooni EA. Chest radiographic and CT findings in novel swine-origin influenza A (H1N1) virus (S-OIV) infection. AJR Am J Roentgenol. 2009; 193:1488–1493.
12. Ajlan AM, Quiney B, Nicolaou S, Muller NL. Swine-Origin Influenza A (H1N1) Viral Infection: radiographic and CT findings. AJR Am J Roentgenol. 2009; 193:1494–1499.
13. Lee EY, McAdam AJ, Chaudry G, Fishman MP, Zurakowski D, Boiselle PM. Swine-Origin Influenza A (H1N1) viral infection in children: initial chest radiographic findings. Radiology. 2010; 254:934–941.
14. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992; 56:152–179.
15. Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009; 360:2605–2615.
16. Condon VR. Pneumonia in children. J Thorac Imaging. 1991; 6:31–44.
17. Wildin SR, Chonmaitree T, Swischuk LE. Roentgenographic features of common pediatric viral respiratory tract infections. Am J Dis Child. 1998; 142:43–46.
18. Griscom NT. Pneumonia in children and some of its variants. Radiology. 1988; 167:297–302.
19. Donnelly LF. Imaging in Immunocompetent children who have pneumonia. Radiol Clin N Am. 2005; 43:253–265.
20. Bramson RT, Griscom NT, Cleveland RH. Interpretation of chest radiographs in infants with cough and fever. Radiology. 2005; 236:22–29.
21. Aherne W, Bird T, Court SDM, Gardner PA, McQillin J. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Path. 1970; 23:7–18.
22. Broughton RA. Infections due to Mycoplasma pneumonia in childhood. Pediatr Infect Dis. 1986; 5:71–85.
23. McCullers JA. Insights into the Interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006; 19:571–582.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download