Abstract
Breast cancer is one of the most common malignancies in women. Breast cancer frequently metastasizes to the bones, lungs, and liver. However, the recurrence of distant soft-tissue metastasis except to the chest wall is extremely rare. Here, we describe our experience with a patient in whom invasive lobular carcinoma of the breast with metastasis to the abdominal wall presented as subcutaneous nodules without local recurrence.
Invasive lobular carcinoma accounts for 5-15% of all breast cancers (1). Moreover, it differs from invasive ductal carcinoma, the most common histologic subtype of breast cancer, not only with respect to histologic and mammographic characteristics, but also in the pattern of metastatic spread (2).
While the spread to the lymph nodes, lung and liver is common in both ductal and lobular carcinomas, invasive lobular carcinoma has been found to metastasize frequently to the gastrointestinal tract, peritoneum and retroperitoneum, and the gynecological organs (345). To the best of our knowledge, no previous cases of distant subcutaneous metastatic nodules from invasive lobular carcinoma, except for chest wall recurrence, has been reported. We report a case of tumor infiltration into the anterior abdominal wall presenting as a palpable mass 3 years after the treatment of an invasive lobular carcinoma of the breast.
A 64-year-old woman presented at our hospital with palpable nodules at the lower and mid abdominal wall that had appeared a month earlier. Three years prior, she underwent a right partial mastectomy and sentinel lymph node (LN) biopsy due to breast carcinoma. Surgical histopathology revealed a 2.2×1.3 cm invasive lobular carcinoma of histologic grade III. No metastasis was found in the axillary lymph node sampling from the sentinel LN biopsy. There was no evidence of distant metastases at the time of surgery. Consequently, the breast cancer was consistent with stage IIb. Following the surgical procedure, she received adjuvant chemotherapy, radiotherapy at a total of 5940 cGy dose, and hormone therapy (aromatase inhibitor). There was neither evidence of recurrence nor metastatic lesions during the follow-up examinations (six monthly ultrasound, mammography, and annual abdominal-pelvic and chest CT) over the three years period after surgery.
Upon admission, the patient presented with hard palpable nodules in the abdomen. However, mammographic findings were noted in Category 2, and the nodules were found to be benign. Sonographic examination (Philips-Advanced Technology Laboratories, Bothell, WA, USA) of the abdominal wall showed a lobulated heterogeneous echoic mass measuring about 2.0×1.4 cm in the subcutaneous layer of the right lower abdominal wall, and a 1.1×0.8 cm sized hyperechoic nodule in the mid abdominal wall (Fig. 1). These lesions were considered as suspicious abnormalities which suggest the presence of metastasis from breast cancer or another malignant lesion (sarcoma). PET/CT scan was performed using F-18 FDG (PET camera; Allegro, Philips, Cleveland, Ohio, CT; Somatom Plus Power, Siemens, Erlangen, Germany). They showed two instances of increased FDG uptakes along the abdominal wall, which corresponded to the subcutaneous nodules on CT scan (Fig. 2). US-guided core-needle biopsy for the nodule in the mid abdominal wall was performed and the pathology revealed the metastatic lesion from breast cancer. A histopathologic examination revealed an invasive lobular carcinoma with a prominent signet ring cell feature (Fig. 3) and the tumor was positive for estrogen but not for progesterone receptors. The result of immunohistochemical staining was negative for E-caderine, but the tumor expressions of HER2 and p53 were positive. The patient received additional chemotherapy.
Some reports suggest that the metastatic spread of invasive lobular carcinoma shows a particular pattern (16). Compared with the behavior of invasive ductal carcinoma, the behavior of an invasive lobular carcinoma is quite different (45). Although invasive lobular carcinoma may metastasize as frequently as invasive ductal carcinoma to the lung, pleura, liver, and lymph nodes, it also has been found to frequently metastasize to an atypical site such as the gastrointestinal tract, peritoneum and retroperitoneum, and gynecological organs (1245). Some studies found that the rates of metastasis (invasive lobular carcinoma vs. invasive ductal carcinoma) to the gastrointestinal system (4.5% vs. 0.2%), gynecologic organs (4.5% vs. 0.8%), peritoneum-retroperitoneum (3.1% vs. 0.6%), adrenal glands (0.6% vs. 0%), bone-marrow (21.2% vs. 14.4%), and lung-pleura (2.5% vs. 10.2%) were documented (4).
Some literature reviews found metastatic lesions of invasive lobular carcinoma presented in the chest wall (2). There was also a report on a case of tumor infiltration into the fasciae of the anterior muscular compartment of the thigh, which presented as an edema in the lower extremity 10 years after the treatment of an invasive lobular carcinoma of the breast (7). Thus, distant metastasis to the soft-tissue metastasis from invasive lobular carcinoma was extremely rare. To our best knowledge, there were no previously reported cases of distant subcutaneous metastasis from invasive lobular carcinoma.
The molecular mechanisms of the different metastatic patterns between invasive ductal carcinoma and invasive lobular carcinoma have not been fully outlined. Nevertheless, it has been reported that the loss of the E-cadherin function, a cell-cell adhesion molecule frequently altered in invasive lobular carcinoma (8), could be related with local and metastatic tumor progression (9). A study reported that in a series of metastatic invasive lobular carcinomas, the complete loss of E-cadherin expression in the primary was documented in 88% of the cases (10). Thus, it is assumed that the down-regulation of E-cadherin might affect or be involved in tumor growth and dissemination condition (9). Lobular carcinoma is a distinct subtype of breast carcinoma that differs from infiltration into ductal carcinoma with a histologic appearance. Clinicians should notice when facing the metastatic relapse of invasive lobular carcinoma, that multiple metastatic sites are likely to be observed in at least 25% of cases. Radiologists often play an integral role in examining patients with lobular carcinoma to evaluate metastasis and to assess treatment response.
In conclusion, we described a case of multiple subcutaneous metastatic nodules in the abdominal wall from an invasive lobular carcinoma of the breast. Invasive lobular carcinoma of the breast can metastasize to the abdominal wall, presenting as a soft tissue mass. The radiologist should consider metastasis from invasive lobular carcinoma of the breast when subcutaneous nodules are present.
Figures and Tables
Fig. 1
A 64-year-old woman with a right partial mastectomy performed 3 year ago.
A. US image demonstrates a lobulated heterogeneous echoic mass measuring about 2.0×1.4 cm in the subcutaneous layer of the right lower abdominal wall (white arrows).
B. Another 1.1 cm large hyperechoic nodule at the mid abdominal wall is seen (white arrows).
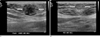
Fig. 2
PET-CT scan of the patient.
A. Increased FDG uptake along the abdominal wall which corresponds to the 2.0 cm sized nodule on CT scan and noted on US image (white arrow)
B. Another FDG uptake lesion is noted at the mid abdominal wall corresponding to the 1.1 cm sized round nodule on CT scan (white arrow).

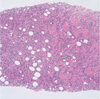
Fig. 3
Photomicrograph of the core biopsy specimen (×100). A photomicrograph of a histologic specimen from abdominal wall mass shows isolated cells and small cords of cells an "Indian file" pattern (arrow) that is characteristic of lobular carcinoma and diffusely infiltrating skeletal muscle fibers (arrowhead) (H and E, × 100).
References
1. Sastre-Garau X, Jouve M, Asselain B, Vincent-Salomon A, Beuzeboc P, Dorval T, et al. Infiltrating lobular carcinoma of the breast: clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer. 1996; 77:113–120.
2. Winston CB, Hadar O, Teitcher JB, Caravelli JF, Sklarin NT, Panicek DM, et al. Metastatic lobular carcinoma of the breast: patterns of spread in the chest, abdomen, and pelvis on CT. AJR Am J Roentgenol. 2000; 175:795–800.
3. Harris M, Howell A, Chrissohou M, Swindell RI, Hudson M, Sellwood RA. A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating duct carcinoma of the breast. Br J Cancer. 1984; 50:23–30.
4. Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery. 1993; 114:637–641.
5. Lamovec J, Bracko M. Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol. 1991; 48:28–33.
6. Kidney DD, Cohen AJ, Butler J. Abdominal metastases of infiltrating lobular breast carcinoma: CT and fluoroscopic imaging findings. Abdom Imaging. 1997; 22:156–159.
7. El Khoury M, Cherel P, Becette V, De Maulmont C, Costes V, Talma V, et al. Unusual soft-tissue metastasis of an invasive lobular carcinoma mimicking fasciitis. AJR Am J Roentgenol. 2004; 182:745–747.
8. Rasbridge SA, Gillett CE, Sampson SA, Walsh FS, Millis RR. Epithelial (E-) and placental (P-) cadherin cell adhesion molecule expression in breast carcinoma. J Pathol. 1993; 169:245–250.
9. Van Aken E, De Wever O, Correia da Rocha AS, Mareel M. Defective E-cadherin/catenin complexes in human cancer. Virchows Arch. 2001; 439:725–751.
10. Ferlicot S, Vincent-Salomon A, Medioni J, Genin P, Rosty C, Sigal-Zafrani B, et al. Wide metastatic spreading in infiltrating lobular carcinoma of the breast. Eur J Cancer. 2004; 40:336–341.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download