Abstract
A Marjolin's ulcer refers to malignancies that developed in chronic venous ulcers, scars, or sinuses. We report three-dimensional computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)-CT findings in a patient who developed skin cancer from a chronic leg ulcer. Although rare, on MR, a Marjolin's ulcer should be considered when a well-enhanced soft-tissue mass with a broad based skin ulcer shows a mass effect and invasion of the adjacent bone. CT angiography and PET-CT complement MRI for evaluating the nature of Marjolin's ulcers and may provide essential anatomical information, enabling the physician to design the optimal surgical approach or determining cancer staging.
The term "Marjolin's ulcer" refers to malignancies that developed in chronic venous ulcers, scars, or sinuses (12). These malignancies also arise from various scars, including chronic ulcerations, inflammation, and fistulas after a long period of latency. Malignant transformation takes approximately 35 years. The incidence of malignant skin tumors that developed from scar tissue is 0.1-2.5%. Burn scars are the most common lesion causing this malignancy (3). The most common malignancy that arises from a Marjolin's ulcer is squamous cell carcinoma, whereas basal cell carcinomas are rare. The malignancy is frequently multiple in the floor of an ulcer (3).
The pathogenesis of a Marjolin's ulcer is controversial (13). Ulcer osteomas develop as a result of the heaping up of periosteum and associated subperiosteal sclerosis, and were seen as a knob-like mass protruding from the bone surface (1). These masses are rare in patients with chronic ulcers (1); however, they are frequently seen in patients with tropical ulcers, occurring in form of cutaneous leishmaniasis, which is a skin infection caused by a single-celled parasite that is transmitted by sandfly bites (4).
A previous report described gadopentetate dimeglumine-enhanced magnetic resonance imaging (MRI) as a useful tool for evaluating a Marjolin's ulcer (2). A multidisciplinary approach using three-dimensional computed tomography (CT) and positron emission tomography (PET)-CT is essential for the imaging workup of malignant cancers for metastatic disease. To our knowledge, the presentation of imaging features of a Marjolin's ulcer as a soft-tissue mass with an ulcer osteoma have seldom been reported in the radiological literature. Here, we report on a case of a Marjolin's ulcer, with an emphasis on a multimodality approach including 3D CT, MRI, and PET-CT.
A 57-year-old woman presented with a 2-month history of a progressively worsening swelling on her left leg and discharge from a scar. She had suffered a flame burn injury 10 years earlier. Subsequently, there had been no marked change in the burn scar on her left leg. However, over the 6 months before her presentation, a mass in the scar had progressively increased in size, and was associated with bloody discharge. Laboratory results were within normal limits.
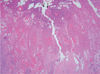
Radiography of the left lower leg showed an ulcer osteoma cortical thickening on the lateral aspect of left fibula, adjacent to the skin ulcer (Fig. 1). Contrast-enhanced CT demonstrated cortical erosion on the ulcer osteoma and a well-enhanced soft-tissue mass with a broad base in the skin ulcer (Figs. 2A, B). CT angiography showed a highly vascular mass supplied by multiple arterial feeders (Fig. 2C). MRI was performed with a 3.0 T MR scanner (Signa HDxt; GE healthcare, Milwaukee, Wis, USA). The soft-tissue mass abutting the skin ulcer showed low signal intensity on T1-weighted images, high signal intensity on fat-saturated T2-weighted images, and strong enhancement on contrastenhanced T1-weighted images (Figs. 3A-D). In addition, fat saturated T2 weighted images showed high signal intensity lesions in bone marrow surrounded by the cortical hypertrophy which showed a patchy enhancement on contrast enhanced fat saturated T1 weighted images. PET-CT showed intense fluorodeoxyglucose (FDG) uptake (standardized uptake value, SUV = 11.2) in the mass, but with no abnormal uptake in the bone marrow adjacent to the skin ulcer (Fig. 4). An excisional biopsy of the soft-tissue mass was performed and the microscopic analysis revealed squamous cell cancer in the soft tissue mass and lymph nodes (Fig. 5). After neoadjuvant chemotherapy, the patient underwent an above-knee amputation based on the CT and MRI findings. The pathological specimen obtained at amputation confirmed tumor involvement in the bone marrow which corresponded to the area with high signal intensity on a T2 weighted image and strong enhancement on a contrast enhanced T1 weighted image (Fig. 3).
An ulcer-osteoma was first described in patients with tropical ulcers from West Africa (1). Periosteal reaction is the earliest radiological finding and is initially present in the bone beneath the ulcer only. Further, the periosteal reaction has a fusiform shape, but occasionally looks like a sunburst. This periosteal new bone eventually fuses with the original cortex, which results in the thickening of the sclerotic cortex by more than 2.5 cm, giving rise to the classical 'ivory ulcer osteoma.'The periosteal reaction may eventually involve the entire circumference of the long bone shaft (4).
In our case, the fusiform cortical hypertrophy beneath the skin ulcer was clearly demonstrated on CT. Karasick et al. (5), whom described two types of periosteal responses to chronic leg ulcers: a solid organized type that forms an ulcer osteoma over time and a lamellar nodular type that is often associated with osteomyelitis. Karasick et al. further indicated that patients with both types of periosteal reaction usually had associated peripheral vascular disease, intravenous drug abuse, sickle cell disease, or neurologic impairment (5). Unlike these previous reports, an ulcer-osteoma adjacent to a skin ulcer occurred in our patient, who had no clinical or laboratory finding of the underlying diseases previously associated with ulcer osteomas (5).
Chiang et al. (2) reported that contrast-enhanced MRI is the imaging technique of choice for assessing a Marjolin's ulcer, because MRI is superior at characterizing the nature and extent of the ulcer (1). In our study, a highly enhanced mass in the skin ulcer site was clearly seen on contrast-enhanced MRI, allowing for the assessment of the margin and depth of the tumor.
Computed tomography has an advantage over MRI for detecting cortical destruction, which helps to determine the relationship between the soft-tissue tumor and adjacent bone (6). With the advent of multi-detector (MD) CT, the use of three-dimensional (3D) CT provides better anatomical resolution for delineating the nature and extent of the bony involvement for preoperative planning (6). As seen in our case, multiplanar CT images with 3D reconstruction demonstrated the extent of the cortical erosion caused by direct extension of the tumor, and the details of the ulcer-osteoma. Furthermore, on CT angiography with volume rendering, multiple arterial feeders were seen supplying the tumor mass; this may help in identifying malignant tumors.
Recently, PET-CT imaging has increasingly been used for the diagnosis and staging of tumors. Studies indicate that 18F-FDG-PET-CT reliably differentiated malignant soft-tissue and bone tumors from benign ones (78), although there was considerable overlap in the maximum SUV value between benign and malignant tumors (9). Shin et al. (7) reported that the sensitivity, specificity, and diagnostic accuracy of 18FFDG-PET-CT were 80%, 68.4%, and 75%, respectively, for soft-tissue tumors with a maximum SUV cutoff of 3.8. In our case, the soft tissue mass had a SUV approximately three times greater than 3.8, strongly suggesting that the tumor was malignant. However, PET-CT did not demonstrate tumor involvement of the bone marrow just below the area of cortical destruction, which was seen in MRI images.
Wide local excision (surgical margin of at least 2 cm) combined with skin grafting is the treatment of choice for a Marjolin's ulcer (10). Amputation is considered only when the lesions involve a joint, invade the limb bones, or there is deep extensive local invasion (2). The roles of routine regional lymph node dissection and irradiation remain controversial (2).
In conclusion, although rare, a Marjolin's ulcer should be considered when a well-enhanced and broad based soft-tissue mass on a skin defect shows mass effects and invasion to an adjacent ulcer osteoma. CT angiography with volume rendering and PET-CT complement MRI when evaluating the nature of Marjolin's ulcer, and may provide essential anatomical information for the surgeon when designing the optimal surgical approach or determination of cancer staging.
Figures and Tables
Fig. 1
Anteroposterior radiograph of the left lower leg shows cortical thickening of the fibular diaphysis (arrowheads) beneath the skin ulcer (arrow). Note the cortical hypertrophy on the opposite side of the skin ulcer.
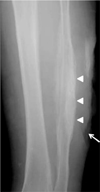
Fig. 2
Computed tomography.
A. Axial CT with bone setting reveals cortical hypertrophy of the left lateral fibula (arrows) extending anteroposteriorly parallel to the skin ulcer (arrowheads). A soft tissue mass (asterisk) is also seen between the skin ulcer and fibula.
B. Sagittal reformatted contrast-enhanced CT shows a thickened cortex (arrows), which is extrinsically eroded by a soft-tissue mass (asterisk) with a broad base on the skin ulcer (arrowheads).
C. CT angiography with color-coded volume-rendering demonstrates a well-enhanced soft-tissue mass (asterisk) surrounded by numerous supplying arteries.
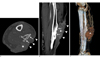
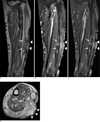
Fig. 3
Magnetic resonance imaging.
A-C. The soft tissue mass (arrowheads) shows slightly greater signal intensity than muscle on (A) coronal T1weighted images [repetition time (TR) 1466, echo time (TE) 15], (B) high signal intensity on fat-saturated T2-weighted images (TR 3550, TE 65), and (C) strong enhancement on contrast enhanced fat saturated T1-weighted images (TR 833,TE 13). Note the cortical involvement of the soft tissue mass and abnormal signal intensity lesions in the bone marrow (arrows).
D. The axial contrast-enhanced T1-weighted image (TR 900, TE 11) shows a highly homogenous enhanced mass bulging out from the skin scar.
References
1. Smith J, Mello LF, Nogueira Neto NC, Meohas W, Pinto LW, Campos VA, et al. Malignancy in chronic ulcers and scars of the leg (Marjolin's ulcer): a study of 21 patients. Skeletal Radiol. 2001; 30:331–337.
2. Chiang KH, Chou AS, Hsu YH, Lee SK, Lee CC, Yen PS, et al. Marjolin's ulcer: MR appearance. AJR Am J Roentgenol. 2006; 186:819–820.
3. Rieger UM, Kalbermatten DF, Wettstein R, Heider I, Haug M, Pierer G. Marjolin's ulcer revisited--basal cell carcinoma arising from grenade fragments? Case report and review of the literature. J Plast Reconstr Aesthet Surg. 2008; 61:65–67.
4. Kolawole TM, Bohrer SP. Ulcer osteoma-bone response to tropical ulcer. Am J Roentgenol Radium Ther Nucl Med. 1970; 109:611–618.
5. Karasick D, Schweitzer ME, Deely DM. Ulcer osteoma and periosteal reactions to chronic leg ulcers. AJR Am J Roentgenol. 1997; 168:155–157.
6. Pretorius ES, Fishman EK. Volume-rendered three-dimensional spiral CT: Musculoskeletal applications. Radiographics. 1999; 19:1143–1160.
7. Shin DS, Shon OJ, Han DS, Choi JH, Chun KA, Cho IH. The clinical efficacy of (18)F-FDG PET/CT in benign and malignant musculoskeletal tumors. Ann Nucl Med. 2008; 22:603–609.
8. Feldman F, van Heertum R, Manos C. 18-FDG PET scanning of benign and malignant musculoskeletal lesions. Skeletal Radiol. 2003; 32:201–208.
9. Aoki J, Watanabe H, Shinozaki T, Takagishi K, Tokunaga M, Koyama Y, et al. FDG-PET for preoperative differential diagnosis between benign and malignant soft tissue masses. Skeletal Radiol. 2003; 32:133–138.
10. Fleming MD, Hunt JL, Purdue GF, Sandstad J. Marjolin's ulcer: a review and reevaluation of a difficult problem. J Burn Care Rehabil. 1990; 11:460–469.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download