Abstract
Traumatic neuromas are rare benign lesions that develop from non-neoplastic proliferation of axons, schwann cells, and fibroblasts at the proximal end of transected or injured nerves as a result of trauma or surgery. We present the case of a traumatic neuroma in a 47-year-old female who was treated with a right modified radical mastectomy for breast cancer 14 years ago. Ultrasound examination revealed an oval-shaped hypoechoic nodule at the 9-o'clock position in the right chest wall. Color Doppler imaging showed no increased blood flow and a positron emission tomography-computed tomography examination revealed no fluorodeoxyglucose uptake in this nodule. The typical histologic findings were present.
Traumatic neuromas are rare benign lesions that develop in the proximal end of injured nerves, and reflect repair of the distal segment (1). The most common location of traumatic neuromas is the lower extremities, followed by the head and neck. Traumatic neuromas arising in post-operative scars after cancer surgery can mimic recurrent lymph nodes or local tumor recurrence. Traumatic neuromas occurring in the chest wall after modified radical mastectomy (MRM) are very rare. We present a case of a traumatic neuroma identified incidentally on follow-up ultrasound (US) examination after breast cancer surgery.
A 47-year-old female who underwent a right MRM at the age of 33 years was referred to our hospital for evaluation of newly developed nodules detected on follow-up US. At that time of diagnosis, the breast cancer stage was T2N0M0 and she underwent a MRM followed by adjuvant chemotherapy for 1 year. At the time of the present admission, the patient was asymptomatic and the physical examination was normal.
The US examination showed an oval, circumscribed, hypoechoic nodule at the 9 o'clock position in the right chest wall with a maximum diameter of 0.8 × 1.0 cm (Fig. 1). A partially microlobulated margin in the lateral and inferior margin of the nodule was noted during the thorough examination (arrows in Figs. 1A and 1B). A color Doppler image showed no increased blood flow within the mass. Because of the patient's history and findings on US, the lesion was suspicious for tumor recurrence. However, a subsequent positron emission tomography (PET)-computed tomography (CT) evaluation revealed no fluorodeoxyglucose (FDG) uptake in the nodule (arrow in Fig. 2). For pathologic confirmation, a surgical excision was performed.
Gross pathologic examination revealed a well-defined, white-gray nodule. Microscopically, the nodule was composed of disordered and irregularly proliferating nerve fascicles surrounded by fibro-adipose tissues (Fig. 3). The final histologic diagnosis was a traumatic neuroma.
Traumatic neuromas are rare, benign lesions that develop from a non-neoplastic proliferation of axons, schwann cells, and fibroblasts at the proximal end of transected or injured nerves as a result of trauma or surgery (2). Rene et al. (1) suggested a pathophysiologic hypothesis of traumatic neuromas from a neural point of view. Accordingly, a neuroma is thought to be an attempt to find a distal segment and complete nerve repair when the distal stump is lost. Nerve proliferation continues from the proximal stump, producing a disorganized tangle of cells composed of neural fibers and connective tissue that extends into surrounding soft tissues (1).
The most common location of traumatic neuromas is the lower extremity after amputation, followed by the head and neck (2). Traumatic neuromas occurring in mastectomy scars are very rare and only three studies have been published (345).
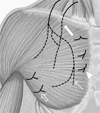
During MRM nerves running around the breast (long thoracic, thoracodorsal, lateral pectoral, medial pectoral, and branches of the intercostals nerves) are susceptible to the transected injury (6). Anatomically, the lateral and medial pectoral nerves, and the anterior and lateral cutaneous branches from the 4th through 6th intercostal nerves are closely located to the pectoralis major muscle with branches passing through the pectoralis major muscle (Fig. 4). The lateral pectoral nerve originates from the C5-6 root and courses inferolaterally through the clavipectoral fascia supplying the lower one-third of the pectoralis major muscle (7). Injury to the lateral pectoral nerve can produce variable post-operative muscle atrophy and change in the cosmetic contour of pectoral region of the chest. Furthermore, because of its anatomic course and variation, the nerve may be compromised during division of the pectoralis muscle and during resection of the central and anterior lymph nodes during a MRM (8).
In our case the traumatic neuroma was located in the outer portion of the right chest wall and the deep portion of the pectoralis major muscle. On FDG PET-CT, we noted mild atrophic changes of the lower outer portion of the right pectoralis major muscle compared with the left side, suggesting the possibility of injury to the lateral pectoral nerve.
The rate of local recurrence at the chest wall following mastectomy ranges between 5% and 27%, and approximately 80% of local recurrences occur within the first 5 years (9). In our case, a traumatic neuroma developed 14 years after a MRM. Wang et al. (5) reported that traumatic neuromas developed > 5 years after mastectomy in 3 of 6 patients.
Although some studies have reported post-operative breast pain associated with traumatic neuromas (6), most cases present as palpable masses without pain. Our patient had no pain and no palpable masses, and the lesion was found incidentally on a follow-up US.
The most common sonographic finding of traumatic neuromas is a well-defined hypoechoic mass. Hyperechoic nerve bundles may be visible running into the mass. Rarely, traumatic neuromas can have irregular or poorly defined margins (10). Wang et al. (5) described traumatic neuromas after mastectomy in eight lesions of six breast cancer patients. Six of the eight lesions had circumscribed margin and two lesions had poorly defined margin. In our case, the nodule had a nearly circumscribed margin; however, even with a thorough examination we only deteected a partially microlobulated margin to the nodule.
In the present case the lesion was found in the deep portion of the pectoralis muscle layer and this may be helpful for the differential diagnosis because most recurrent lesions in the mastectomy bed after MRM occur in the subcutaneous fat layer. However, chest wall invasion may also occur in recurrent breast cancer by direct local extension of the tumor through the pectoral fascia and into the pectoral muscles or by indirect extension via the interpectoral nodes.
In conclusion, it is very difficult to diagnose traumatic neuromas in breast cancer patients after MRMs because there are is overlap in the US findings between recurrences and traumatic neuromas. However, if a color Doppler image shows no increased blood flow, FDG PET-CT shows no significant uptake, and the time interval between surgery and the development of new lesion is more than 5 years, one should include traumatic neuromas in the differential diagnosis.
Figures and Tables
Fig. 1
Ultrasound examination.
A. Longitudinal sonogram of the 9 o' clock position of the right chest wall shows an oval-shaped, hypoechoic mass with a circumscribed and partially microlobulated margin (arrow).
B. Transverse sonogram also reveals a partially microlobulated margin (arrow). This nodule is located within the pectoralis muscle layer.
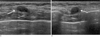
Fig. 2
Axial scan of PET-CT reveals no focal uptake area in the outer portion of the right chest wall (arrow). We can see mild atrophic changes of the outer portion of the right pectoralis major compared with the left side.
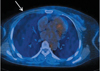
Fig. 3
Microscopic findings.
A. Microscopic specimen of the breast lesions shows disordered and irregularly proliferating nerve fascicles (arrows) surrounded by fibro-adipose tissues (hematoxylin eosin stain, original magnification ×100).
B. At higher magnification, some hyperplastic nerve bundles are composed of axons, schwann cells, and fibroblasts (original magnification ×400).
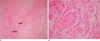
Fig. 4
Schematic drawing showing anatomic location of nerves around the pectoralis muscle.
Lateral and medial pectoral nerves, and lateral and anterior cutaneous branches from intercostal nerves are closely located with the pectoralis major muscle. Lateral pectoral nerve (arrow) originates from the C5-6 roots, moves inferolaterally through the clavipectoral fascia, and supplies inferior and lateral portion of the pectoralis major. Medial pectoral nerve (large arrow) originates from C8-T1 and mainly enters the deep surface of the pectoralis minor muscle. Lateral (open arrow) and anterior (arrow head) cutaneous branches of the intercostal nerve run on the pectoralis major muscle and supply the skin and mammary tissue.
References
1. Foltan R, Klima K, Spackova J, Sedy J. Mechanism of traumatic neuroma development. Med Hypotheses. 2008; 71:572–576.
2. Murphey MD, Smith WS, Smith SE, Kransdorf MJ, Temple HT. From the archives of the AFIP. Imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlation. Radiographics. 1999; 19:1253–1280.
3. Rosso R, Scelsi M, Carnevali L. Granular cell traumatic neuroma: a lesion occurring in mastectomy scars. Arch Pathol Lab Med. 2000; 124:709–711.
4. Baltalarli B, Demirkan N, Yagci B. Traumatic neuroma: unusual benign lesion occurring in the mastectomy scar. Clin Oncol (R Coll Radiol). 2004; 16:503–504.
5. Wang X, Cao X, Ning L. Traumatic neuromas after mastectomy. ANZ J Surg. 2007; 77:704–705.
6. Wong L. Intercostal neuromas: a treatable cause of postoperative breast surgery pain. Ann Plast Surg. 2001; 46:481–484.
7. Provencher MT, Handfield K, Boniquit NT, Reiff SN, Sekiya JK, Romeo AA. Injuries to the pectoralis major muscle: diagnosis and management. Am J Sports Med. 2010; 38:1693–1705.
8. Moosman DA. Anatomy of the pectoral nerves and their preservation in modified mastectomy. Am J Surg. 1980; 139:883–886.
9. Yilmaz MH, Esen G, Ayarcan Y, Aydogan F, Ozguroglu M, Demir G, et al. The role of US and MR imaging in detecting local chest wall tumor recurrence after mastectomy. Diagn Interv Radiol. 2007; 13:13–18.
10. Beggs I. Sonographic appearances of nerve tumors. J Clin Ultrasound. 1999; 27:363–368.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download