Abstract
Giant cell tumors are benign osteolytic tumors with a variable degree of aggressiveness. We report a rare case of a giant cell tumor involving the metacarpal bone, which was detected during pregnancy and showed rapid progression on a follow-up examination.
Giant cell tumors (GCT) of bone are benign, locally aggressive tumors that account for 4.0-9.5% of all primary bone neoplasms (12). GCT predominates in the long tubular bones, but does, in rare cases, occur in the small bones of the hands and feet. Most GCT cases occur at the sacrum, with only very rare cases occurring during pregnancy (34). We report a case of a GCT involving a metacarpal bone with rapid growth during pregnancy, which was misdiagnosed as a malignant bone tumor on plain radiographs.
A 32-year-old woman at 32 weeks gestation was evaluated for swelling of the left 4th finger. The clinician told her that the swelling was a non-specific pregnancy-related symptom and did not perform an imaging assessment. However, she visited another clinic 1 month later at 36 weeks gestation due to a worsening of the swelling and associated limitation of finger movement. She underwent plain radiographs and magnetic resonance (MR) imaging of the left hand. The radiographs revealed an expansile osteolytic mass with cortical destruction in the central portion of the meta-epiphysis of the left 4th metacarpal bone (Fig. 1A). MR imaging of the lesion in the sagittal view revealed a lobulated mass with soft tissue extension and adjacent soft tissue edema (Fig. 2). The mass showed low-to-intermediate signal intensity on T1-weighted images, and heterogeneous intermediate-to-high signal intensity on fat-suppressed T2-weighted images. Additionally, multiple foci of dark signal intensity were evident in all sequences within the mass. Contrast-enhanced MR imaging showed heterogeneous enhancement with a non-enhancing region in the central portion of the mass. Intra-articular extension into the metacarpophalangeal joint via a disrupted joint capsule and involvement of adjacent soft tissue was noted.
The patient was transferred to our hospital at 38 weeks gestation (2 weeks after the initial radiograph) for further evaluation and management of the mass and underwent a repeat hand radiograph (Fig. 1B). The mass showed an increased extent of bone destruction and an aggressive periosteal reaction in a speculated or elevated pattern, suggesting the possibility of a malignant mass, such as an osteosarcoma, considering the aggressive growth pattern of the mass. On the next day, she underwent a cesarean section, as well as a surgical biopsy of the mass. The GCT diagnosis was made on the histopathologic examination, with multinucleated giant cells and mononuclear cells.
A bone scan was performed 4 days after the biopsy and demonstrated peripherally increased radionuclide uptake with central photopenia. No other bony lesion was detected. A pre-operative radiography and computed tomography (CT) of the hand were performed the day before surgical excision (3 weeks after delivery, and 5 weeks after the initial radiograph). The radiograph showed further progression of the tumor with severe destruction of bone (Fig. 1C). A sagittal CT reconstruction image revealed a relatively well-marginated, expansile osteolytic mass with cortical bone destruction in the distal metacarpal bone (Fig. 3). The size of the osteolytic mass and extent of the accompanying soft tissue mass were markedly increased, compared with the previous MR imaging. The periosteal reaction was expansile and had a very thin, curvilinear appearance. In the proximal portion of the lesion, an irregularly elevated periosteum and partially discontinuous periosteal reaction were noted, with partly preserved cortical bone. Thinning and penetration of the articular surface was noted at the metacarpal head. Moreover, the 4th proximal phalanx was intact and no evidence of intratumoral mineralization was discovered.
The excision of the mass with an osteochondral allograft was performed. The mass was a relatively well-encapsulated mass, measuring 4 × 3 × 3 cm. The cut surface was a red-brown and gray, heterogeneous, fresh mass containing some bony tissue. No malignant cells were detected, and in the immunohistochemical assay of the specimen, no estrogen or progesterone receptors were demonstrated.
GCT of bone are frequently found at the distal femur, proximal tibia, and distal radius (1). Involvement of the bones of the hand, as in our case, is rare and accounts for 2-4% of cases (2). Tumors most frequently occur in the metaphyseal side of the epiphysis of long tubular bones, predominantly affecting young adults after closure of the growth plate (1). Tumors are found as geographic osteolytic lesions, eccentrically located in long tubular bones, and centrally located in short tubular bones (15). A sclerotic rim or periosteal reaction is relatively unusual on radiography, but frequently noted on CT (1). Aneurysmal bone cysts are present in 14% of GCT (1). On MR imaging, the tumor has a low-to-intermediate signal intensity on T1-weighted image and heterogeneous high signal intensity on T2-weighted image. Very low signal intensity within the tumor is frequently seen on all pulse sequences, representing intratumoral hemosiderin deposition (1). GCT can exhibit a locally aggressive and invasive appearance with cortical penetration accompanied by soft tissue invasion or a pathologic fracture (1). Although it is rare, malignant transformation or metastases to the lung is also possible (1). Local recurrence of the tumor is frequent, occurring in 40-60% of all cases (1). There have been reports that GCT in hand bones tend to show early involvement of the entire bone with more aggressive behavior and multicentricity (26). In our case, the serial plain radiographs showed marked rapid growth, as well as a very aggressive appearance with an irregularly spiculated or elevated pattern of periosteal reaction in part, which led to the misdiagnosis of a malignant bone tumor. The MR appearance of the tumor could not exclude the possibility of a malignancy. On CT, however, a well-defined, expansile mass with a partly discontinuous, but very thin shell-like periosteal reaction and focally preserved cortical bone was noted. These CT findings favor the locally aggressive, but benign nature of GCT.
A few published articles have described the combination of pregnancy and a primary bone tumor (4789). Bone tumors during pregnancy tend to be missed or diagnosed after progression of the tumor because the symptoms can be mistaken as a pregnancy-related symptom (4). The diagnosis of GCT occurring in the sacrum, the most frequently involved site in pregnant women, is often delayed because tumor-related symptoms are falsely attributed to pregnancy (4). Painful swelling, as in our patient with GCT involving the metacarpal bone, was overlooked at the initial clinic evaluation.
Osteosarcomas, chondrosarcomas, and giant cell tumors have been reported as tumors in pregnant women (479). The influence of pregnancy on the occurrence, promotion, and development of these tumors is unclear. In cases of GCT, there were several articles which studied the endocrinologic and immunologic mechanisms to explain the high incidence of GCT during pregnancy with respect to hormonal receptors on the tumor cells, such as estrogen or progesterone receptors, but they still remain controversial (410). Oncofetal antigens, which are present on tumor cells and are shared with or resemble fetal antigens, may explain tumor promotion during pregnancy (410). In the case of osteosarcomas, the tumor contains high levels of heat-sensitive alkaline phosphatase as one of the oncofetal antigens (4). However, in the case of GCT, no oncofetal antigens have been reported (4).
There have been a few reports related to the occurrence or progression of GCT during pregnancy, but there have been no studies with respect to the rapid aggravation of tumor in such a short follow-up period, as in this case. Also, as far as we know, the relationship between pregnancy and tumor progression rate has not been studied. In our case, the result of the immunohisochemical study was negative for estrogen and progesterone receptors. This supports the idea which Turcotte et al. (3) advanced, that it may be coincidental to find GCT in pregnant women because affected patients are often of childbearing age, the age in which tumors show a high incidence in the general population.
In summary, we report a rare case of a giant cell tumor in the metacarpal bone which demonstrated very rapid progression, an aggressive appearance during pregnancy, and the mimicking of a malignant bone tumor.
Figures and Tables
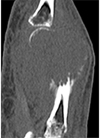
Fig. 1
Serial radiographs of the left hand.
A. Posteroanterior radiographs at 36 weeks gestation (initial study): an expansile, osteolytic mass with cortical destruction is noted in the meta-epiphysis of the left 4th metacarpal bone.
B. The radiograph obtained 2 weeks after the initial film shows an increase in size of the mass and an aggressive periosteal reaction at the proximal portion of the lesion.
C. The pre-operative radiograph obtained 5 weeks after the initial film reveals more extensive bone destruction with extension to the articular surface.
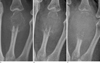
Fig. 2
Fat-suppressed (FS) T2-weighted (A) and FS contrast-enhanced T1-weighted (B) sagittal MR images obtained at 36 weeks gestation (along with Fig. 1A radiograph). The mass is shown as a heterogeneous intermediate-to-high signal intensity on a FS T2-weighted image, and an irregular-enhancing peripheral portion with non-enhancing in the central portion of the mass. Multiple intratumoral dark signal intensities throughout both sequences are noted. The mass extends into the intra-articular portion of the metacarpophalangeal joint and adjacent soft tissues.
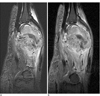
Fig. 3
A sagittal reconstruction image of the computed tomogram for the pre-operative work-up (along with the Fig. 1C radiograph). A relatively well-defined, expansile osteolytic, mass without intratumoral mineralization is noted. A periosteal reaction is noted as generally very thin, curvilinear, and partly discontinuous. Note the aggressive appearance with an irregular elevated periosteum, but preservation of the adjacent cortical bone in the proximal portion of the mass.
References
1. Murphey MD, Nomikos GC, Flemming DJ, Gannon FH, Temple HT, Kransdorf MJ. From the archives of afip. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologicpathologic correlation. Radiographics. 2001; 21:1283–1309.
2. James SL, Davies AM. Giant-cell tumours of bone of the hand and wrist: a review of imaging findings and differential diagnoses. Eur Radiol. 2005; 15:1855–1866.
3. Turcotte RE, Sim FH, Unni KK. Giant cell tumor of the sacrum. Clin Orthop Relat Res. 1993; 215–221.
4. Komiya S, Zenmyo M, Inoue A. Bone tumors in the pelvis presenting growth during pregnancy. Arch Orthop Trauma Surg. 1999; 119:22–29.
5. Minhas MS, Mehboob G, Ansari I. Giant cell tumours in hand bones. J Coll Physicians Surg Pak. 2010; 20:460–463.
6. Kotnis NA, Davies AM, Kindblom LG, James SL. Giant cell tumour of the triquetrum. Skeletal Radiol. 2009; 38:593–595.
7. Maxwell C, Barzilay B, Shah V, Wunder JS, Bell R, Farine D. Maternal and neonatal outcomes in pregnancies complicated by bone and soft-tissue tumors. Obstet Gynecol. 2004; 104:344–348.
8. Ross AE, Bojescul JA, Kuklo TR. Giant cell tumor: a case report of recurrence during pregnancy. Spine. 2005; 30:E332–E335.
9. Simon MA, Phillips WA, Bonfiglio M. Pregnancy and aggressive or malignant primary bone tumors. Cancer. 1984; 53:2564–2569.
10. Ishibe M, Ishibe Y, Ishibashi T, Nojima T, Rosier RN, Puzas JE, et al. Low content of estrogen receptors in human giant cell tumors of bone. Arch Orthop Trauma Surg. 1994; 113:106–109.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download