Abstract
Angiosarcoma of the chest wall is a very rare tumor and it is difficult to radiologically differentiate this tumor from other malignant tumors. Chronic tuberculous empyema is a predisposing factor that has been associated with angiosarcoma. We report here on a case of a 66-year-old man with angiosarcoma that arose in the chest wall. Computed tomography (CT) demonstrated a heterogeneous enhancing mass in the chest wall with calcified pleural thickening and multiple pulmonary nodules with the halo sign, which all indicated the presence of sarcoma with hypervascular metastases.
Angiosarcoma is an uncommon tumor and it accounting for approximately 1% of all soft tissue tumors. Angiosarcoma is a neoplasm that arises from endothelial cells and it usually originates in the small blood vessels. It can affect virtually any organ, but it most commonly develops in the skin and deep soft tissues, liver, spleen, heart and breast (1). We present here a rare case of primary angiosarcoma of the chest wall that was associated with chronic empyema and hypervascular pulmonary metastases. To the best of our knowledge, there has been no case report on the computed tomography (CT) and positron emission tomography-computed tomography (PET-CT) features of a patient with angiosarcoma of the chest wall with hypervascular pulmonary metastases.
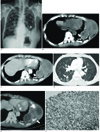
A 66-year-old man presented with chest tightness, pain and a palpable mass that he'd had for several months. He showed mild dyspnea at the time of admission. He had a 30-year history of chronic tuberculous empyema. A chest radiograph showed a large mass in the left lower chest wall and multiple ill-defined pulmonary nodules in both lungs (Fig. 1A). The axial CT scans were obtained with using 16-channel multidetector CT after the intravenous administration of contrast media. The contrast-enhanced chest CT demonstrated a well-defined lobulated mass that measured 11.5 × 9 cm in diameter with mild heterogeneous enhancement, and it arose in the left chest wall. The mean density of the solid portion of the mass was 31 Hounsfield units (HUs) on the precontrast scan and 43 HUs on the postcontrast scan with non-enhancing low density areas. This mass was associated with calcified thickened parietal pleura, proliferated extrapleural fatty tissue and multiple destroyed adjacent ribs, and the tumor extended through the diaphragm with invasion of the superior portion of the spleen (Figs. 1B, C). The lung setting image showed multiple small nodules each with a surrounding halo of ground glass opacity in both lungs (Fig. 1D). PET-CT was performed after 10.3 mCi F-18 fluoro-deoxy-glucose (FDG) administration to identify the metastatic sites. Multiple focal areas of increased uptake were seen in the peripheral portion of the mass, the sternum, ribs and the vertebral bodies (Fig. 1E). The maximum standard uptake value (maxSUV) of the solid portion of the mass was 4.55.
Surgery was not an option because of the extent of the disease. Careful biopsies were taken from the left lateral portion of the chest wall tumor and the pulmonary nodules. The microscopic examinations revealed the same results that the tumor was poorly differentiated, it produced no definite blood vessels and it had the appearance of dense clumps of irregular anaplastic cells. Immunohistochemically, the tumor cells were strongly positive for CD31 (Fig. 1F) and vimentin, while they were negative for cytokeratin, desmin, CD56, TTF-1 and smooth muscle actin. Vascular tumor can be highly suspected when the immunohistochemical staining for cytokeratin is negative and that for vimentin is positive. CD31 is a highly specific, sensitive endothelial marker that reacts rarely and only weakly with nonvascular tumors. So, these were characteristic and specific findings of angiosarcoma. The pathologic diagnosis was angiosarcoma, which was in accordance with the radiologic findings.
The patient underwent chemoradiation treatment. A follow-up CT scan obtained six months after the diagnosis showed newly developed bony metastases and progression of the primary mass and the pulmonary metastases.
Primary sarcomas of the thorax are rare. Angioarcoma is a sarcoma with a predominantly intrathoracic location such as the cardiac and mediastinal sarcomas rather than having a chest wall location (2). Angiosarcomas originating from the pleura and chest wall are extremely rare.
The pathogenesis and etiology of angiosarcoma of the chest wall are not clear. It may be caused by a long-standing inflammatory process, chronic stimulation of mesothelial cells or the action of oncogenic substances contained in the pleura. The predisposing factors that have been implicated include trauma, chronic lymphedema, radiation, foreign bodies, thorium, viral infection and chronic empyema (3). The histopathologic diagnoses of the reported cases associated with chronic empyema have been malignant lymphoma, squamous cell carcinoma, mesothelioma, malignant fibrous histiocytoma, liposarcoma and angiosarcoma. The mean duration of chronic empyema before the diagnosis of malignancy has been reported to be about 25 years (4). Such a predisposing factor was present in our patient.
Primary malignant chest wall tumors typically manifest as large, palpable, rapidly growing masses. The rare empyema-associated malignant tumors that need to be considered in the differential diagnosis of angiosarcoma include lymphoma, malignant fibrous histiocytoma and squamous cell carcinoma. However, not much information has appeared in the literature with regard to the specific radiologic manifestations of these tumors. The imaging features of many malignant chest wall tumors are nonspecific (5). The specific feature of chronic empyema-associated lymphoma is a symmetric growth pattern of a mass at the margin of the chronic empyema (6). The reported CT findings of angiosarcoma reveal a nonspecific soft tissue chest wall mass with attenuation similar to that of muscle or a circumferential lobular pleural mass with various degrees of enhancement after the intravenous injection of contrast medium (78). In our case, CT demonstrated a large well-defined lobulated mass with mild heterogeneous enhancement around the calcified thickened parietal pleura, which suggested the occurrence of malignancy in the background of chronic empyema. CT also demonstrated multiple small nodules each with a surrounding halo of ground glass opacity in both lungs. This appearance is usually associated with hemorrhagic nodules. Many infectious, neoplastic and inflammatory processes cause this halo sign (9). However, if this presents together with hypervascular tumor such as angiosarcoma, choriosarcoma, osteosarcoma and melanoma, then multiple pulmonary nodules with a halo of ground glass opacity suggest the presence of lung metastases. Angiosarcoma may be distinguished from other tumors by a history of chronic empyema along with hypervascular metastasis.
PET with 18F-FDG may be used for the staging of angiosarcoma. Some reports have shown focal, intense accumulation of FDG in angiosarcomas of the chest wall, pleura, breast and liver, with an SUV up to 7.5 (710). In our case, PET-CT demonstrated multiple focal areas of increased uptake in the peripheral portion of the chest wall mass, with no evidence that the pulmonary nodules were hypermetabolic. We suggest that the pulmonary nodules showed no significant uptake of FDG because the size of the solid portion of the nodules was less than 7 mm.
We have described the CT and PET features of angiosarcoma and pulmonary metastasis. A mass in the chest wall that is associated with chronic empyema and multiple pulmonary nodules with the halo sign suggests the presence of angiosarcoma with hypervascular metastases.
Figures and Tables
Fig. 1
A 66-year-old man with angiosarcoma of the chest wall and pulmonary metastases.
A. The chest radiograph shows an extrapulmonary mass (arrow) in the left lower chest wall and multiple ill-defined pulmonary nodules (arrowheads) in both lungs.
B, C. The axial CT images before (B) and after contrast injection (C) display a lobulated mass associated with calcified thickened pleura, proliferated extrapleural fatty tissue (arrow) and destroyed adjacent multiple ribs, and the mass is extending into the chest wall.
D. The lung setting image at the level of the bronchus intermedius shows multiple pulmonary nodules each with a halo sign (arrow) in the right lung.
E. The PET-CT images show multiple focal areas of increased uptake in the peripheral portion of the tumor (arrow).
F. Immunohistochemically, the tumor cells are strongly positive for CD31 (×100).
References
1. Patel AM, Ryu JH. Angiosarcoma in the lung. Chest. 1993; 103:1531–1535.
2. Gladish GW, Sabloff BM, Munden RF, Truong MT, Erasmus JJ, Chasen MH. Primary thoracic sarcomas. Radiographics. 2002; 22:621–637.
3. Aozasa K, Naka N, Tomita Y, Ohsawa M, Kanno H, Uchida A, et al. Angiosarcoma developing from chronic pyothorax. Mod Pathol. 1994; 7:906–911.
4. Minami M, Kawauchi N, Yoshikawa K, Itai Y, Kokubo T, Iguchi M, et al. Malignancy associated with chronic empyema: radiologic assessment. Radiology. 1991; 178:417–423.
5. Tateishi U, Gladish GW, Kusumoto M, Hasegawa T, Yokoyama R, Tsuchiya R, et al. Chest wall tumors: radiologic findings and pathologic correlation: part 2. Malignant tumors. Radiographics. 2003; 23:1491–1508.
6. Ueda T, Andreas C, Itami J, Miyakawa K, Fujimoto H, Ito H, et al. Pyothorax-associated lymphoma: imaging findings. AJR Am J Roentgenol. 2010; 194:76–84.
7. Del Frate C, Mortele K, Zanardi R, Hunsaker AR, Nikpoor N, Cibas ES, et al. Pseudomesotheliomatous angiosarcoma of the chest wall and pleura. J Thorac Imaging. 2003; 18:200–203.
8. Sugita R, Takezawa M, Itinohasama R. Primary angiosarcoma of the chest wall: CT and MR findings. Radiat Med. 2002; 20:101–103.
9. Pinto PS. The CT Halo Sign. Radiology. 2004; 230:109–110.
10. Maeda T, Tateishi U, Hasegawa T, Ojima H, Arai Y, Sugimura K. Primary hepatic angiosarcoma on coregistered FDG PET and CT images. AJR Am J Roentgenol. 2007; 188:1615–1617.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download