Abstract
A 38-year-old man was admitted to our hospital with diplopia. The patient had a relatively well-defined pituitary mass with high cellularity as well as weaker enhancement on imaging modalities including computed tomography (CT) and magnetic resonance imaging (MRI), than a typical pituitary adenoma. The distinction between a pseudotumor and an invasive neoplasm is very difficult before biopsy. In this case report, we discuss the characteristic imaging features of a fibrosing inflammatory pseudotumor of the pituitary gland.
An inflammatory pseudotumor of the pituitary gland, which is usually preoperatively diagnosed as a pituitary adenoma, is a very rare non-neoplastic lesion. We report a case of a fibrosing inflammatory pseudotumor located in the pituitary gland, that presented with abducens nerve palsy and was confirmed by histopathological analysis. Knowledge of the imaging features of inflammatory pseudotumors can help avoid unnecessary radical surgery prior to histopathological proof of malignancy.
A 38-year-old man presented with an eight month history of anorexia, sexual dysfunction, a visit to a private clinic; however, his symptoms were not improving despite conservative treatment. Abrupt-onset ophthalmoplegia and diplopia developed for 7 days prior at admission to the neurosurgery ward of our hospital. A neurologic exam revealed limitation of the extraocular muscle during left lateral gaze with aggravated diplopia, suggesting left 6th cranial nerve palsy. Biochemical studies revealed the hormonal profile of panhypopituitarysm.
CT images revealed a homogeneously high attenuated box-shaped mass, involving the sellar, parasellar (including cavernous sinuses), and retrosellar regions with homogeneous enhancement and some bony erosion of the dorsum sella, which extended to the sphenoid sinus (Fig. 1). T2-weighted MR images showed localized relatively homogeneous low signal intensity mass at the sellar, parasellar, and retrosellar regions. T1-weighted MR images showed iso-signal intensity of the lesion. On gadolinium enhanced T1-weighted images, homogeneously weak enhancement of the mass with involvement of the bilateral cavernous sinuses was observed along with contour-bulging. However, there was no demonstrable suprasellar bulging (Fig. 2). A left ICA angiogram showed tumor staining on the venous phase (Fig. 3A), as well as focal severe stenosis at the left distal cavernous ICA on the arterial phase (Fig. 3B). The differential diagnoses included invasive pituitary adenoma, meningioma, lymphoma, and germ cell tumor.
The patient underwent transnasal-transsphenoidal surgery where a pink-colored, soft tissue was partially removed, and was surrounded by fibrous tissue. A normal pituitary gland could not be identified separately and no evidence of intratumoral hemorrhage or necrosis was seen.
A histopathologic examination of the surgical specimen revealed tissue fragments consisting mainly of collagenous dense fibrous tissue proliferation with infiltration of multiple inflammatory cells (mainly plasma cells, lymphocytes and some histiocytes) and a few normal adenohypophysial cells; however, no tumor cells were identified (Fig. 3C). An immunohistochemical study revealed that several cells were immunoreactive in their cytoplasma for EMA (plasma cell marker) and CD 68 (histiocyte marker). Microbiological studies were performed, but no specific microorganism was isolated. After pathologic diagnosis, the patient took antibiotics for 2 months and did well postoperatively on replacement doses of hydrocortisone and synthyroid. Finally, after 3 years, postoperative follow-up MRI showed shrinkage of the sellar mass.
Inflammatory pseudotumors of the head and neck most commonly occur in the orbits, or more rarely in the skull base (1). Inflammatory pseudotumors of the pituitary gland are a very rare non-neoplastic lesion (2). To our knowledge, fewer than 3 inflammatory pseudotumor cases located in the pituitary gland have been reported in the literature to date (234). We report a case of an inflammatory pseudotumor in the pituitary gland, which was confirmed by histopathological and immunohistochemical analysis.
A fibrosing inflammatory pseudotumor involving the pituitary gland can mimic a pituitary tumor or invasive fungal disease, and the distinction between pseudotumor and pituitary neoplasm is very difficult without a biopsy (5). Therefore sophisticated diagnostic procedures are necessary to provide an accurate diagnosis in the case of infectious or inflammatory etiology, which may lead to a nonaggressive and efficient medical therapy (3).
The term inflammatory pseudotumor represents an inflammatory lesion with a broad spectrum of histological features, varying from a predominance of inflammatory cells mixed with some fibrous tissue, to extensive fibrous lesions trapping some inflammatory cells (6). It has been reported that all 18 pathologically confirmed cases of inflammatory pseudotumor involving CNS demonstrated contrast enhancement, little or no mass effect, and little or no peritumoral edema (7). Also, all 5 cases of tumors involving the skull base demonstrated homogeneous contrast enhancement and hypointensity of the lesion on T2-weighted images, which seemed to be related to the degree of fibrosis (8). The lack of mobile protons due to the fibrotic background and/or high cellularity of the lesions may be the reason for their hypointensity and weaker enhancement on MR images (8).
The etiology of inflammatory pseudotumors remains obscure; an autoimmune hypothesis is thought to be the most likely (3). Moreover, the treatment of inflammatory pseudotumors is controversial. Many researchers agree that accessible lesions should be first treated surgically (39). When surgery cannot be performed or is incomplete, corticosteroids may be administered with a greater efficacy in pure inflammatory lesion than in fibrous lesions (10).
In our case, ophthalmoplegia and diplopia during the left lateral gaze could be explained by the cavernous sinus involvement. High cellularity of the lesion may be the reason for high attenuation on CT images and hypointensity with diffuse relatively homogeneous, weak enhancement on T2-weighted MR images. Though size of the mass is relatively large, there was no evidence of compression of the optic chiasm due to negligible suprasellar extension of the mass, which seems to be different from the imaging feature of a typical pituitary adenoma. In addition, the visual acuity and visual fields were normal. A left ICA angiogram showed weak tumor staining on the venous phase compared with tumor staining of meningioma, as well as focal severe stenosis in the left distal cavernous ICA in the arterial phase, as like meningioma or invasive fungal disease, which seems to be the result of mixed inflammatory and some tumefactive nature of this lesion.
In conclusion, an inflammatory pseudotumor in the pituitary gland shows characteristic CT and MR findings with hyperattenuation on CT images, homogeneous low signal intensity on T2-weighted images, homogeneously weak enhancement on CT, MR images, no demonstrable suprasellar bulging contour, and weak tumor staining on the venous phase of angiograms. If the mass or lesion involves the pituitary gland and parasellar region with characteristic findings, an inflammatory pseudotumor should be included in the differential diagnosis, and a surgical intervention is necessary and important for proper treatment of the disease.
Figures and Tables
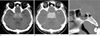 | Fig. 1A 38-year-old man presenting with ophthalmoplegia and diplopia that aggravated the left lateral gaze.A. Pre-contrast CT image showing a well defined, homogeneously high attenuated lesion in the sella and parasellar regions.
B. Postcontrast CT image shows homogeneously well enhanced lesion with encasement of both cavernous ICA.
C. Sagittal bone algorithm CT image showing permeative bone erosion of the sellar floor and dorsum sella (arrows).
|
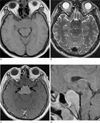 | Fig. 2A. T1-weighed axial MR image showing a well defined iso-signal intensity lesion, involving the sellar and parasellar region.B. T2-weighted axial MR image showing a well defined low signal intensity lesion, involving the sellar and parasellar region.
C. Contrast-enhanced T1-weighted axial MR image showing homogenous enhancement of the lesion with bilateral involvement of the cavernous sinuses associated with encasement of the both cavernous ICA (arrows).
D. Contrast-enhanced T1-weighted sagittal MR image shows the caudal portion of the lesion extending to the retrosellar region. Note that minimal suprasellar bulging contour of the mass was evident despite the involvement of both cavernous sinuses.
|
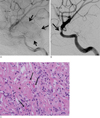 | Fig. 3A. Left ICA angiogram showing tumor staining in the venous phase, similar to a meningioma, but with a weaker degree of demarcation than staining of the meningioma.B. Left ICA angiogram showing focal severe stenosis of the Lt. distal cavernous ICA in the arterial phase.
C. Photomicrograph of the inflammatory pseudotumor (inflammatory myofibroblastic tumor) of the pituitary TSA specimen showing underlying collagenous dense fibrous tissue (asterisk) which was infiltrated by prominent inflammatory infiltrates (mainly plasma cells, lymphocytes, and histiocytes) with a Russel body (small, pink colored, spherical intracytoplasmic hyaline body, arrows) (H-E stain, magnification, × 400).
|
References
1. Lee JH, Kim KJ, Chung SW, Choi YC, Lee Ah. A case report of inflammatory pseudotumor involving the clivus : CT and MR findings. Korean J Radiol. 2001; 2:231–234.
2. Al-Shraim M, Syro LV, Kovacs K, Estrada H, Uribe H, Al-Gahtany M. Inflammatory pseudotumor of the pituitary gland: case report. Surg Neurol. 2004; 62:264–267.
3. Hansen I, Petrossians P, Thiry A, Flandroy P, Gaillard RC, Lovacs K, et al. Extensive inflammatory pseudotumor of the pituitary. J Clin Endocrinol Metab. 2001; 86:4603–4610.
4. Murakami K, Muraishi K, Ikeda H, Yoshimoto T. Plasma cell granuloma of the pituitary gland. Surg Neurol. 2001; 56:247–251.
5. Choi SY, Yu IK, Han MH, Lee BH, Song CJ, Kim KS. Fibrosing inflammatory pseudotumor of the nasopharynx: MR features and histopathologic correlation. Euro J Radiol. 2009; 72:274–277.
6. Mombaerts I, Goldschmeding R, Schlingemann RO, Koornneef L. What is orbital pseudotumor? Surv Ophthalmol. 1996; 41:66–78.
7. Swain RS, Tihan T, Horvai AE, Di Vizio D, Loda M, Burger PC, et al. Inflammatory myofibroblastic tumor of the central nervous system and its relationship to inflammatory pseudotumor. Hum Pathol. 2008; 39:410–419.
8. Han MH, Chi JG, Kim MS, Chang KH, Kim KH, Yeon KM, et al. Fibrosing inflammatory pseudotumors involving the skull base : MR and CT manifestations with histopathologic comparison. AJNR Am J Neuroradiol. 1996; 17:515–521.
9. Seider MJ, Cleary KR, Van Tassel P, Alexanian R, Schantz SP, Frias A, et al. Plasma cell granuloma of the nasal cavity treated by radiation therapy. Cancer. 1991; 67:929–932.
10. Char DH, Miller T. Orbital pseudotumor. Fine needle aspiration biopsy and response to therapy. Ophthalmology. 1993; 100:1702–1170.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download