Abstract
We illustrate the sonographic findings of malignant breast masses that can mimic a cystic component with pathologic correlations. The disease entities presented in this study include infiltrating ductal carcinoma, ductal carcinoma in situ (DCIS), papillary carcinoma, mucinous carcinoma, medullary carcinoma, metaplastic carcinoma, and a malignant phyllodes tumor. Malignant masses with a cystic component are often characterized by well-circumscribed round, oval, or lobular masses, thereby appearing benign on ultrasonography. On pathology, the cystic component of a malignant mass is identified by cystic degeneration, hemorrhage, necrosis, or ductal dilatation. If the mass is well-circumscribed with a cystic component, a biopsy should be considered in the analysis of the solid component within a mass.
An important function of breast ultrasonography is the differentiation of a cyst from a solid lesion (12). Cystic breast lesions may be associated with numerous pathological entities, but malignant cystic breast masses are rare. Mixed cystic and solid carcinomas of the breast are uncommon findings and constitute 0.3% to 2.0% of all breast carcinomas (23). There are three situations in which a cystic lesion can be associated with malignancy described as an invasion of a carcinoma into an area of cystic disease, cystic degeneration of a high-grade malignancy, and the presence of an intracystic papillary carcinoma (3). Berg et al. (2) described the sonographic and pathologic correlations of cystic lesions of the breast in which 23% (18/79) of the complex cystic masses were found to be malignant. It is suggested that 67% (12/18) of sonographically circumscribed cystic masses were malignant and that sonography may be helpful in identifying those circumscribed masses that merit a biopsy. A malignant cystic mass of the breast can be seen with a circumscribed margin on imaging. Circumscribed cancers of the breast are listed as infiltrating ductal carcinomas not otherwise specified (IDC-NOS), mucinous carcinomas, medullary carcinomas, papillary carcinomas, phyllodes tumors and adenoid cystic carcinomas (4). Imaging findings of various malignant cystic breast masses with pathological correlations are illustrated. Knowledge of the disease spectrum, as well as the characteristics of malignant cystic masses, may be helpful in the selection of whether to perform a biopsy as part of patient management.
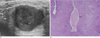
Central necrosis of a high-grade infiltrating ductal carcinoma can lead to a complex cystic mass in the breast. Subsequent hemorrhage into the site of necrosis will lead to the formation of a large hemorrhagic malignant cyst (3). An infiltrating ductal carcinoma is seen as a hypoechogenic mass with irregular margins and variable acoustic shadowing on sonography, but it will appear as a well-circumscribed mass with posterior acoustic enhancement (Fig. 1A) (1). A residual solid mass or bloodtinged fluid following aspiration is suggestive of malignancy and a biopsy should be performed. Pathologic central necrosis of a high-grade infiltrating ductal carcinoma can lead to a cystic component within a solid mass (Fig. 1B) (3).
Ductal carcinoma in situ (DCIS) is a proliferation of malignant ductal epithelial cells with no evidence of invasion of basement membrane on pathology. DCISs are made up of a heterogeneous group of lesions with variable histologic features. On mammography, DCIS is typically depicted as microcalcifications, although it may also appear as a mass without microcalcifications (5). The most common sonographic findings in DCIS without calcifications are a solid and cystic mass or a large mass with posterior enhancement. Lesions associated with DCIS can also be misinterpreted as benign nodules due to their round and well-circumscribed margins (Fig. 2A) (5). The pathologic analysis of these lesions are described non-comedo type lesions, (i.e., of cribriform, micropapillary, papillary, or solid composition) (Fig. 2B). Intracystic papillary carcinoma is considered as a variant of papillary type DCIS in which tumor cells are located primarily in a single cystically dilated ductal space (5).
Papillary carcinoma accounts for fewer than 2% of all breast cancers and can be further classified as papillary carcinoma in situ and infiltrating papillary carcinoma. Clinically, a papillary carcinoma is often accompanied by nipple discharge or a palpable mass (6). If the cystic component is present, the tumor is described as an intracystic papillary carcinoma. The most common mammographic pattern of invasive papillary carcinoma is a round, oval, or lobulated mass, usually with a circumscribed margin. Ultrasonographic evaluation of invasive papillary carcinomas may reveal a hypoechogenic, solid mass with posterior acoustic enhancement or a complex cystic and solid mass, or a cystically dilated space with papillary projection of mural nodularity (Fig. 3A) (6). These lesions usually undergo US-guided aspiration and a core needle biopsy, but these lesions have been surgically excised as a papillary carcinoma and cannot be distinguished from a papilloma. On pathology, intracystic papillary carcinoma is formed from dilated intramammary ducts and papillary growths (6). The tumor is a central fibrovascular core lined by markedly proliferated monotonous epithelial cells with dilatation of the mammary duct (Fig. 3B) (67). Cystic regions may represent the imaging correlate of the representative hemorrhagic and cystic areas seen on a pathological evaluation.
Mucinous (colloid) carcinoma of the breast is an uncommon carcinoma and represents 1 to 7% of all invasive breast carcinomas and predominantly affects older women. Histopathologically, mucinous carcinomas can be divided into pure or mixed mucinous carcinomas based on mucinous content. The pure type has a better prognosis. Mammographic appearance is different in pure and mixed mucinous types. A well-defined mass with lobulation is characteristic of pure mucinous carcinoma and a poorly defined or spiculated margin is characteristic of mixed mucinous carcinoma (7). The ultrasonographic findings of mucinous carcinomas are complex cystic and solid masses, which have increased vascularity and distal enhancement. A pure mucinous carcinoma may present as a circumscribed, homogeneous hypo/isoechoic mass and the lesion can be considered as a circumscribe-margined carcinoma (Fig. 4A). This feature is probably the result of the high water content and transmission of the ultrasound beam through the mucin and is therefore more commonly found in tumors with a high -mucin content. On pathology, anechoic areas of mass are not truly cystic, but represent mucin with floating malignant cells (Fig. 4B) (7).
Medullary carcinoma accounts for up to 7% of all breast carcinomas and tends to occur in women less than 35 years of age. Medullary carcinoma is characterized by rapid growth, and lesions are detected as a palpable mass. Medullary carcinoma is often characterized by well-circumscribed masses with a predilection for areas of cystic degeneration, appearing benign on mammography and ultrasonography (Fig. 5A). Typical medullary carcinoma has better circumscribed borders than in the atypical medullary carcinoma. The irregular margins are consistent with the presence of carcinoma infiltrates on pathology and are more common in atypical medullary carcinoma (8). The pathologic diagnostic criteria for typical medullary carcinoma include complete syncytial growth, poorly differentiated nuclear grade, inflammation, well-defined tumor margins, intraductal components, and glandular features (Fig. 5B). Medullary cancer may look cystic, but they are packed with cells and there are no cystic cavities upon pathologic examination. The prognosis of medullary carcinoma is more favorable than infiltrating ductal carcinoma NOS.
Metaplastic carcinoma occurs in less than 5% of breast carcinomas with both carcinomatous and sarcomatous features that arise from non-glandular mesenchymal tissues. Moreover, it has many different histopathological changes including spindle cell carcinoma, carcinoma with osseous metaplasia, carcinoma with psedosarcomatous metaplasia, squamous cell carcinoma with pseudosarcomatous stroma, fibrosarcoma-like squamous cell carcinoma, and carcinosarcoma. Metaplastic carcinoma usually presents in women older than 50 years as a rapidly growing, palpable mass. On mammography, a metaplastic carcinoma has been described as a predominantly circumscribed, non-calcified mass with a spiculated portion. On US, microlobulated, complex echogenic masses with solid and cystic components may be seen (Fig. 6A) (9). The cystic components are related to necrosis and cystic degeneration and are discovered histopathologically (Fig. 6B) (9).
The phyllodes breast tumor is a special kind of fibroadenoma that constitutes 0.3-1.0% of all breast tumors. However, in contrast to other fibroadenomas, phyllodes tumors have the potential to grow to a large size in middle-aged to older women and have a high incidence of local recurrence if not completely removed. Phyllodes tumors may become extremely large and lesions 3 cm or greater in diameter have a higher likelihood of malignancy (10). The appearance of phyllodes tumors is similar to that of fibroadenomas on ultrasonography, but is occasionally seen with large, oval, relatively circumscribed complex echogenic masses that are more common in phyllodes tumors (Fig. 7A). Phyllodes tumors may be classified as benign, low, or high grade or as low, or high grade according to the histological assessment of the malignant potential of the phyllodes tumor and depends on the stromal cellularity, pleomorphism, nuclear atypia, and the growth of stroma. The presence of cysts and hemorrhage are supposed to be an effect of rapid growth and size, with regressive changes occurring in large tumors (Fig. 7B). A phyllodes tumor requires complete surgical excision with wide margins, owing to a high recurrence rate, even for a low-grade tumor. High-grade phyllodes tumors may contain sarcomatous elements. The prognosis is poor for lesions containing a sarcomatous component (10).
Knowledge of the spectrum of disease of malignant breast masses that can mimic a cystic component may be helpful in the differential diagnosis of breast disease. Malignant masses that can mimic a cystic component are often characterized by well-circumscribed round, oval, or lobular masses, thereby appearing benign on ultrasonography. A biopsy should be considered for wellcircumscribed masses if they have cystic a component.
Figures and Tables
Fig. 1
Invasive ductal carcinoma with cystic degeneration.
A. Sonography showed a large ill-defined cystic mass (arrow) with a thick septa and a solid nodular component.
B. Photography (original magnification, × 12.5; H-E stain) revealed pleomorphic malignant cells and hemorrhagic necrosis (arrow).

Fig. 2
Ductal carcinoma in situ (DCIS).
A. Sonography demonstrated multiple conglomerated oval masses within an eccentric cystic component (arrow).
B. Photography (original maaagnification, × 40; H-E stain) revealed DCIS of the micropapillary type. The cystic lesions were lined by micropapillary cells and the lumen was filled with necrotic materials (arrow).
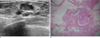
Fig. 3
Papillary carcinoma: aspiration yielded bloody fluid, and following excision biopsy, revealed a low-grade intraductal papillary carcinoma.
A. Sonography scan showed a well-circumscribed, oval cystic mass with thick septa and an eccentric solid nodular component (arrow). A fluid-debris level was seen (arrowhead).
B. Photography (original magnification, × 100; H-E stain) revealed a dilated duct filled with papillary carcinoma (arrow).
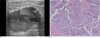
Fig. 4
Mucinous carcinoma.
A. Sonography presented a lobulated, oval mass with an eccentric cystic component (arrow).
B. Photography (original maaagnification, × 40; H-E stain) revealed malignant cells floating in pools of mucin. The tumor cells were present as small papillary clusters with pleomorphic features. The borders were relatively well circumscribed, and the mucinous pools often mimicked cysts (arrow).
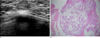
Fig. 5
Medullary carcinoma.
A. Sonography showed hypoechoic cystic lesions (arrow) with a smooth and well-circumscribed margined mass.
B. Photography (original magnification, × 40; H-E stain) showed medullary carcinoma circumscribing lymphoid tissue with multifocal microcystic degenerations (arrow).
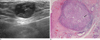
Fig. 6
Metaplastic carcinoma.
A. Sonography demonstrated a microlobulated, oval mass with eccentric cystic components (arrow).
B. Photography (original magnification, × 40; H-E stain) revealed a metaplastic carcinoma of the breast, which showed spindle and squamoid differentiation. The center of the lesion revealed cystic degeneration by necrosis (arrow).
References
1. Venta LA, Dudiak CM, Salmon CG. Sonographic evaluation of the breast. Radiographics. 1994; 14:29–50.
2. Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology. 2003; 227:183–191.
3. Omori LM, Hisa N, Ohkuma K. Breast masses with mixed cysticsolid sonographic appearance. J Clin Ultrasound. 1993; 21:489–495.
4. Harvey JA. Unusual breast cancers: useful clues to expanding the differential diagnosis. Radiology. 2007; 242:683–694.
5. Moon WK, Myung JS, Lee YJ, Park IA, Noh DY, Im JG. US of ductal carcinoma in situ. Radiographics. 2002; 22:269–280.
6. Wagner AE, Middleton LP, Whitman GJ. Intracystic papillary carcinoma of the breast with invasion. AJR Am J Roentgenol. 2004; 183:1516.
7. Lam WW, Chu WC, Tse GM, Ma TK. Sonographic appearance of mucinous carcinoma of the breast. AJR Am J Roentgenol. 2004; 182:1069–1074.
8. Liberman L, LaTrenta LR, Samli B, Morris EA, Abramson AF, Dershaw DD. Overdiagnosis of medullary carcinoma: a mammographic-pathologic correlative study. Radiology. 1996; 201:443–446.
9. Gunhan-Belgen I, Memis A, Ustun EE, Zeikioglu O, Ozdermir N. Metaplastic carcinoma ot the breast: clinical, mammographic, and sonographic findings with histopathologic correlation. AJR Am J Roentgenol. 2002; 178:1421–1425.
10. Liberman L, Bonaccio E, Hamele-Bena D, Abranson AF, Cohn MA, Dershaw DD. Benign and malignant phyllodes tumors: mammographic and sonographic findings. Radiology. 1996; 198:121–124.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download