Abstract
Purpose
The anatomy of the left atrium (LA) and the pulmonary veins (PVs) is important in planning and performing successful electrophysiologic ablation (EPA) for atrial fibrillation (Afib) patients. The authors estimated the findings of LA and PVs of Afib patients by MDCT, and compared these with the findings of LA and PVs of the non-Afib group using coronary CT angiography (CCTA).
Materials and Methods
From September, 2009 to February, 2010, 91 Afib patients underwent PVCT (male: female = 72:19, mean age = 55.0-years-old) before EPA. At same time, 90 patients underwent CCTA (male: female = 73:17, mean age = 59.1-years-old). Two radiologists reviewed and analyzed all axial and 3D images of LA and PVs retrospectively with consensus.
Results
The average LA volumes of the Afib group(100.49 mm3) was larger than that of the non-Afib group (78.38 mm3) (p<0.05). The average lengths of the LA right wall in the Afib group (40.25 mm) was longer than that of the non-Afib group (37.3 mm) (p<0.05). The average distances between the PV ostium and first segmental bifurcation of the Lt superior PV (LSPV) and the RSPV were shorter in the Afib group (LSPV, 19.38 mm; RSPV, 11.49 mm) than in the non-Afib group (LSPV, 23.23 mm; RSPV, 14.25 mm) (p<0.05). There were higher incidences of anomalous branches such as ostial, accessory branches, or common ostia in the Afib group versus the non-Afib group (p<0.05).
Atrial brillation (Afib), the rapid discharge of multiple electrical impulses from ectopic foci, is one of the most common sustained cardiac arrhythmias with significant morbidity and mortality, The muscular sleeves of the distal pulmonary veins (PVs) are a frequent source of ectopic foci of electrical activity (12). Electrophysiologic ablation (EPA) of the distal PVs and posterior left atrium (LA) is a helpful treatment for persistent Afib in patients with pharmacologic therapy or cardioversion (12).
The anatomy of the LA and the PVs of an individual Afib patient is complex, and neither fluoroscopy nor echocardiography can adequately depict it (13). Currently a three-dimensional multi-detector CT (MDCT) of the LA and distal PVs is an extremely useful modality that can provide the necessary intra-and extraatrial anatomic information (12).
Many studies have reported the variability of important anatomical findings using CT with contrast enhancement (12456). Some of these findings include the number, location, and angulation of PVs and their ostial branches as well as variability of LA volume. However, there is a paucity of data about the differences of Afib patients with those of control groups by MDCT (7). Therefore, we evaluated and analyzed the anatomical findings of LA and the PVs in Afib patients and normal patients using CT.
From September 1, 2009 to February 28, 2010, 91 Afib patients underwent with pulmonary vein CT (PVCT) (male: female = 72:19, mean age = 55.0-years-old) before EPA. Ninety non-Afib patients, at high risk for coronary arterial disease or with atypical chest pain underwent coronary CT angiography (CCTA) (male: female = 73:17, mean age = 59.1-years-old). Any patients with a previous history of renal impairment, hypertension, cardiovascular procedure or surgery, pulmonary hypertension (primary or secondary), valve disease, or any kind of cancer in the thorax were excluded from the study. Results of echocardiography in both groups showed no cardiac functional impairment. All CT studies were performed with a 64-multidetector CT (Brilliance 64, Philips Medical System, Cleveland, OH, USA) using this injection protocol: 60 cc~80 cc of iodine contrast media (Ultravist 370, Bayer-Schering Pharma, Berlin, Germany) was injected using 5 cc/sec as an injection rate, followed immediately by 40 cc saline flushing with a 4 cc/sec injection rate. CT protocol for CCTA were as follows: scan range from carina to diaphragm, slice thickness 0.67 mm, slice increment 0.33 mm, 110 Hounsfield unit of scan ROI tracker threshold, 7 sec post injection delay, 7 sec post threshold delay. CT protocol for PVCT were: scan range from aortic arch to diaphragm, slice thickness 1 mm, slice increment 0.5 mm, 200 Hounsfield unit of scan ROI tracker threshold, 10 sec post injection delay, 7 sec post threshold delay. Two observers individually evaluated all images. PVCT was performed without electrocardiogram (ECG) gating. Analysis categories for Afib and non-Afib groups were defined as follows: 1) LA volume, 2) the longest diameter of ostium of LA appendage (LAA), 3) the volume of LAA, 4) the length of superior and inferior roofs of LA, 5) the length of right and left walls of LA, 6) the length of bilateral carina of PV, 7) the diameter of each ostium of PVs, and 8) the distance between PV ostium and 1st segmental bifurcation (Fig. 1). The wall was from the most superior edge of superior PV to the most inferior edge of inferior PV, and the carina was the shortest diameter between the ostia of superior and inferior PVs at same side. Anomalous branch categories of PVs such as accessory branch, common trunk and ostial branch of PV were also evaluated. Evaluation of shortening in the main portion of the PV, an esophageal location behind the LA posterior wall was included. According to the heart rate of non-Afib group, analysis of CCTA was done in mid-diastolic phase (heart rate <65 bpm) or in end-systolic phase (heart rate>65 bpm), to get the best image quality and minimize the image blurring.
Retrospective analysis and review of the 3D LA images was performed with an imaging processing workstation (Aquarius, Terarecon Inc., San Mateo, CA, USA). Two radiologists were reviewed the images without any information with consensus (kappa coefficient = 0.75). The curvilinear lengths on LA were measured according to the linear ablation sites: bilateral antral ablation line as PV ostium, roof lines as superior and inferior roofs, left lateral isthmus line as carina, anterolateral and anteroseptal lines as bilateral walls. Since the LA appendage and the LA have different anatomical characteristics, separate analysis of the LA appendage and the LA was performed in reference to the points of inflection on the 3D CT image (7). The absolute and relative volumes of each portion were calculated and compared.
Statistically, the T-test and Mann-Whitney were used to compare each parameter in the two groups. Anomalous PV branches were evaluated using Fisher's exact test.
We evaluated 181 CT images and the analyzed data are presented in Table 1. The LA volumes of the Afib group were larger than of the non-Afib group (mean: 100.49 mm3 vs. 78.38 mm3, p < 0.05). However, no significant differences were demonstrated for ostial diameter or volume of the LA appendage between the two groups.
The lengths of the superior and the inferior roofs were also similar between the two groups. The length of the LA right wall in the Afib group was longer than in the non-Afib group (mean: 40.25 mm3 vs. 37.30 mm, p < 0.05), but the length of the LA left wall showed no difference between two groups. On the right side, the length of the carina was greater in the Afib group than the non-Afib group (mean: 12.07 mm3 vs. 9.55 mm, p < 0.05), but the lengths of the left side were similar.
In the Afib group, the diameters of the ostia of the RSPV, the RIPV, and the LIPV were significantly larger than those in the non-Afib group (p < 0.05). However, no significant difference was demonstrated for the LSPV ostium. The distances between the PV ostium and the first segmental bifurcation of the LSPV and the RSPV were shorter in the Afib group than in the non-Afib group (p < 0.05).
There was a higher incidence of anomalous branches such as ostial, accessory branches, as well as a greater number of common ostia in the Afib group (57%) versus the non-Afib group (27%) (p < 0.05). The incidence of right, left or both common ostia of the PV was not significantly different between the two groups. There were no significant differences of shortening in the main portion of the PV, an esophageal location behind the LA posterior wall. In the non-Afib group, there were 24 patients with significant coronary stenosis, 39 with nonsignificant coronary stenosis, and 27 with normal coronary artery.
Atrial enlargement is the most common structural change associated with Afib. The Framingham study estimated that the hazard ratio for subsequent development of Afib was 1.39 for every 5-mm incremental increase of LA size by echocardiogram (8). Also, other study suggested that Afib could cause LA dilation (9) and reported that the late recurrence of Afib is associated with progressive dilation of LA (3). Our results showed that the LA size was larger in the Afib patients than in the non-Afib group, which was consistent with the previous studies.
Other studies using transesophageal echocardiography and MDCT reported that the size of the ostium of LAA in Afib patients is larger than in healthy people (101112). The larger LAA size was related to the higher incidence of LAA thrombi. This finding may indicate a higher risk of thromboembolic events in Afib patients (12). In the present study the diameter of LAA ostium showed no statistical difference between Afib patients and the non-Afib group. However, the LAA diameter demonstrates greater variation in Afib patients than in the the non-Afib group. Wongcharoen et al. (12) reported that a longer LA roof and variation in the LA roof morphologies were noted in the Afib patients. In our study, the lengths of the superior and inferior roofs showed no significant difference between the two groups.
Remarkably little has been published concerning normal PV anatomy (13). It is rare to know the PV anatomy of Afib patients before procedures are conducted. Lin et al. (14) were the first investigators to evaluate specifically the PV size in patients with Afib using contrast venography. They showed that the diameters of the RSPV and LSPV were greater than that of the inferior PVs. In a subsequent article, these authors used MRI to evaluate PV anatomy and reported that the superior PVs were larger than the inferior PVs and that the superior, but not the inferior PVs, were larger in diameter in patients with Afib (15). We thought it is plausible that the stretch and dilation of the superior PVs might change the electrophysiologic characteristics of the myocardial sleeve, so that Afib may occur more easily. On the other hand, dilation of the inferior PVs is less significant than in the superior PVs, which may explain in part the lower incidence of Afib initiated from ectopic foci in the inferior PVs. Kato et al. (1213) reported that the diameters of the four PVs do not differ, and that there was little variation in the size of the PVs within given patients. In our study, the diameters of the RSPV, RIPV and LIPV in Afib patients were larger than in the non-Afib group.
PVs exhibit a great deal of anatomical variation. Previous reports described a 23-38% frequency with which variations in PV anatomy were generally observed (1316). Accessory branches and common trunks were described in these studies. These variations of PV anatomy have not been thoroughly evaluated in Afib patients. In our study, we included accessory branches, common trunks, and ostial branches in the anomalous branch category. A significantly higher incidence of anomalous branching was noted in Afib patients compared to the control group. Especially the ostial branch was more common in Afib patient. It is a very interest result.
Other parameters that we assessed, such as the length of LA right and left walls, the length of LA bilateral carina and the distance between the PV ostium and the first segmental bifurcation, had never before been evaluated in any previous study. Our results showed that some parameters differed between the two study groups. Further studies are needed to clarify the clinical significance of these findings.
Several other anatomical differences between Afib patients and normal patients were previously discussed. Chaiang et al. (17) reported that the LA isthmus was longer in the Afib patients and that the morphology of the isthmus was variable. They also compared the lateral isthmus to the medial isthmus finding the latter to be longer and having more ridges. They explained the regional difference as a result of the orientation and thickness of myocardial fibers between the structures of LA. Also Wongcharoen et al. (12) said the LA roof and the LA septum had variation in Afib patients. These anatomical variations are important to consider in ablation therapy, but the pathophysiology and any relationship with clinical outcome have not been revealed.
Several limitations of this study should be considered. First, the PVCT images of Afib group were not always acquired during the period of the cardiac cycle when there was maximal dilation of the LA, due to arrhythmia. However, changes of LA morphology and size are probably minimal between the systolic and diastolic phases in Afib patients. Second, the non-Afib group included patients with normal sinus rhythm who had undergone CCTA. Third, the male/female ratios differed between the two groups. Men probably have larger hearts than women, due to body size.
In contrast to previous studies using MRI or echocardiography, our study was performed using MDCT. A larger number of Afib patients were examined in our study than in previous studies and we evaluated a wider variety of anatomical differences than previous studies. In our results, we detected multiple anatomical differences between Afib patients and the control group. Some of these results were consistent with those of previous studies, but some were not. The incidence of anomalous branches of PV was significantly higher in Afib patients than in the control group. Changes in multiple anatomical parameters that are are presumed to be related to the development, progress and recurrence of Afib. However, there is inadequate information to clearly demonstrate any such relationship. Further studies to investigate this question are currently in progress.
Figures and Tables
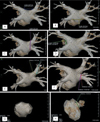
Fig. 1
Measurements of parameters in Afib group. (A) Superior roof, (B) inferior roof, (C) right lateral wall, (D) left carina, (E) and (F) ostium of PV, and diameter between ostium and first segmental bifurcation, (G) LA volume, (H) ostium of LA appendage. Volume of the ostium of the LA appendage was measured as: volume of (H) - volume of (G).
References
1. Stanford W, Breen JF. CT evaluation of left atrial pulmonary venous anatomy. Int J Cardiovasc Imaging. 2005; 21:133–139.
2. Lacomis JM, Wigginton W, Fuhrman C, Schwartzman D, Armfield DR, Pealer KM. Multi-detector row CT of the left atrium and pulmonary veins before radio-frequency catheter ablation for atrial fibrillation. Radiographics. 2003; 23 Spec No:S35–S48.
3. Tsao HM, Wu MH, Huang BH, Lee SH, Lee KT, Tai CT, et al. Morphologic remodeling of pulmonary veins and left atrium after catheter ablation of atrial fibrillation. J Ccardiovasc Electrophysiol. 2005; 16:7–12.
4. Lacomis JM, Goitein O, Deible C, Schwartzman D. CT of the pulmonary veins. J Thorac Imaging. 2007; 22:63–76.
5. Piorkowski C, Hindricks G, Schreiber D, Tanner H, Weise W, Koch A, et al. Electroanatomic reconstruction of the left atrium, pulmonary veins, and esophagus compared with the "true anatomy" on multislice computed tomography in patients undergoing catheter ablation of atrial fibrillation. Heart Rhythm. 2006; 3:317–327.
6. Centonze M, Del Greco M, Nollo G, Ravelli F, Marini M, Della Sala SW, et al. The role of multidetector CT in the evaluation of the left atrium and pulmonary veins anatomy before and after radio-frequency catheter ablation for atrial fibrillation. Preliminary results and work in progress. Technical note. Radiol Med. 2005; 110:52–56.
7. Jongbloed MR, Dirksen MS, Bax JJ, Boersma E, Geleijns K, Lamb HJ, et al. Atrial fibrillation: multi-detector row CT of pulmonary vein anatomy prior to radiofrequency catheter ablation--initial experience. Radiology. 2005; 234:702–709.
8. Dittrich HC, Pearce LA, Asinger RW, Mcbride R, Webel R, Zabalgoitia M, et al. Left atrial diameter in nonvalvular atrial fibrillation: an echocardiographic study. Am Heart J. 1999; 137:494–499.
9. Sanfilippo AJ, Abascal VM, Sheehan M, Oertel LB, Harrigan P, Hughes RA, et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation. 1990; 82:792–797.
10. Li YH, Lai LP, Shyu KG, Hwang JJ, Kuan P, Lien WP. Clinical implications of left atrial appendage flow patterns in nonrheumatic atrial fibrillation. Chest. 1994; 105:748–752.
11. Verhorst PM, Kamp O, Visser CA, Verheugt FW. Left atrial appendage flow velocity assessment using transesophageal echocardiography in nonrheumatic atrial fibrillation and systemic embolism. Am J Cardiol. 1993; 71:192–196.
12. Wongcharoen W, Tsao HM, Wu MH, Tai CT, Chang SL, Lin YJ, et al. Morphologic characteristics of the left atrial appendage, roof, and septum: implications for the ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006; 17:951–956.
13. Kato R, Lickfett L, Meininger G, Dickfeld T, Wu R, Juang G, et al. Pulmonary vein anatomy in patients undergoing catheter ablation of atrial fibrillation: lessons learned by use of magnetic resonance imaging. Circulation. 2003; 107:2004–2010.
14. Lin WS, Prakash VS, Tai CT, Hsieh MH, Tsai CF, Yu WC, et al. Pulmonary vein morphology in patients with paroxysmal atrial fibrillation initiated by ectopic beats originating from the pulmonary veins: implications for catheter ablation. Circulation. 2000; 101:1274–1281.
15. Tsao HM, Yu WC, Cheng HC, Wu MH, Tai CT, Lin WS, et al. Pulmonary vein dilation in patients with atrial fibrillation: detection by magnetic resonance imaging. J Cardiovasc Electrophysiol. 2003; 12:809–813.
16. Ho SY, Sanchez-Quintana D, Cabrera JA, Anderson RH. Anatomy of the left atrium: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 1999; 10:1525–1533.
17. Chiang SJ, Tsao HM, Wu MH, Tai CT, Chang SL, Wongcharoen W, et al. Anatomic characteristics of the left atrial isthmus in patients with atrial fibrillation: lessons from computed tomographic images. J Cardiovasc Electrophysiol. 2006; 17:1274–1278.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download