Abstract
There are no previous reports of germinoma primary arising in the lateral ventricle. We describe here a case of a 50-year-old man with lateral ventricular germinoma that showed rapid progression, and this tumor mimicked choroid plexitis.
Germinomas represent 0.5 to 2.1% of all intracranial tumors and the usual location for intracranial germinomas is the midline structures such as the pineal and suprasellar regions (1). To the best of our knowledge, intracranial germinoma primary arising in the lateral ventricle has not yet been reported in the medical literature. We describe here a case of lateral ventricular germinoma that showed rapid progression and it mimicked choroid plexitis.
A 50-year-old man presented with dizziness and headache. The neurologic examinations showed no abnormal findings except for obtundation. There was no evidence of visual symptoms, pyramidal tract signs or ataxia. The initial brain computed tomography demonstrated mild enlargement of the choroid plexus in the trigone of the right lateral ventricle.
A brain magnetic resonance imaging (MRI) (Fig. 1) obtained four days later demonstrated the enlarged choroid plexus (Fig. 1A) and adjacent ependymal enhancement (Fig. 1B) in the trigone of the right lateral ventricle. On the fluid attenuated inversion recovery (FLAIR) MR images, high signal intensities were noted in the septum pellucidum (Fig. 1C) and along the trigone of the right lateral ventricle. There was also suspicious ependymal enhancement in the left lateral ventricle (Fig. 1B) and mild enlargement of the choroid plexus in the left (Fig. 1A) and fourth ventricles (Fig. 1D). There was no abnormal lesion in the pineal or suprasellar regions (Fig. 1D). Three weeks later, the patient complained of nausea, vomiting and dizziness. The unenhanced brain MR images showed deterioration of the high signal intensities along the trigone of the right lateral ventricle, and diffuse high signal intensities were noted around all the parts of the ventricular system.
Examination of the cerebrospinal fluid (CSF) revealed an increased white blood cell (WBC) count (440/mm3) with 59% lymphocytes, increased adenosine deaminase activity (ADA) (76 IU/l, normal range: 0-10), increased total protein (4,230 mg/dL, normal range: 15-45) and a decreased glucose level (32 mg/dL, 50-70), but the CSF was negative for tuberculous polymerase chain reaction (PCR), herpes simplex virus (HSV) and malignant cells. The serum C-reactive protein (CRP) level and erythrocyte sedimentation rate (ESR) were increased. Despite two weeks of treatment with antibiotics and antituberculous medications, the patient continued to experience severe headache, nausea, vomiting and a decreased level of consciousness.
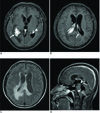
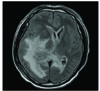
A brain MR images (Fig. 2) obtained five weeks after admission showed more progression of the dense enhancement of the enlarged choroid plexus and the adjacent ependymal enhancement in the right lateral ventricle (Fig. 2A). Obvious ependymal enhancement and/or dense enhancement of the enlarged choroid plexus were noted in the left and fourth ventricles (Fig. 2A, 2B, and 2D). New enhancing lesions appeared in the right lateral ventricle (Fig. 2B), the pineal region (Fig. 2D) and the anterior wall of the fourth ventricle (Fig. 2D). There was progression of the high signal intensities around all the parts of the ventricular system, and especially along the right lateral ventricle (Fig. 2C). MRI performed seven weeks after admission showed extensive white matter edema and midline shifting to the left (Fig. 3).
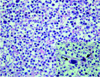
Decompressive craniectomy and transcortical choroid plexus biopsy were performed and histological examination revealed that the tumor consisted of primitive germ cells with an infiltration of small lymphoid cells (Fig. 4). Some of the germ cells showed mitotic figures and giant cells (Fig. 4). The serum levels of human chorionic gonadotropin, alpha-fetoprotein and carcinoembryonic antigen were all within the normal ranges. We planned chemotherapy and radiotherapy, but the general condition of the patient was too poor for treatment to be started. He died due to dissemination 15 weeks after symptom onset.
Ten percent of intracranial germinomas occur in off-midline locations such as the basal ganglia, thalamus or internal capsule (12). Ventricular development may cause migration of ectopic germ cells from the midline, which could explain the occurrence of germinomas in the off-midline structures (3). The lateral ventricle can be involved in midline germinomas through CSF dissemination (4) because germinoma may easily spread through the flow of the CSF due to its nonencapsulation (5). There has been a report of a case of recurrent lateral ventricular germinoma that primary arose in the pituitary gland, which was outside of the initially administered radiation field (6). However, intracranial germinoma primary arising in the lateral ventricle has not been reported. On the initial MRI, ependymal spread and CSF dissemination were suspected because of the suspicious ependymal enhancement and/or the enlarged choroid plexus in the left lateral and fourth ventricles, and these lesions were definitely seen on the follow up MR images. But these suspicious lesions were difficult to differentiate from the normally enhancing choroid plexus and ependymal veins on the initial MRI. In contrast, the enlarged choroid plexus and ependymal enhancement were obviously noted in the trigone of the right lateral ventricle.
Contrary to a previous report (7), our patient had rapid tumor progression. Kobayashi et al. (7) reported that the patients with intracranial germinoma had not received chemotherapy or radiotherapy longer than 16 months from the time of symptom onset because the diagnosis had been delayed. However, those patients showed a good response to the chemotherapy and/or radiotherapy, and they were still alive at the time of publishing the study. In contrast, our patient underwent rapid progression of the lateral ventricular lesion and there was ependymal spread and CSF dissemination with extensive white matter edema, and he died within 15 weeks from symptom onset.
In terms of aggressiveness, there has been a reported case similar to ours. A patient with spinal germinoma underwent six surgical interventions and chemotherapy and/or radiotherapy due to repeated tumor recurrences, and the patient died with seeding around the brain stem (8). That report suggested the possibility of the positive relationship between the high proliferative index and the aggressive nature of this tumor because germinoma is by definition a primitive tumor, and it expected to have a high proliferative index (8). The cause of the rapid progression of our case is uncertain, but it may be explained that some of the germ cells showed mitotic figures on the histopathology.
The choroid plexus may serve as a main portal for hematogenously borne pathogens into the CNS, and it is an important site for the initial dissemination of various pathogens such as tuberculosis, cryptococcosis, nocardiosis and other bacteria and parasites due to lack of a blood-brain barrier (9). On MRI, choroid plexitis shows dense enhancement of the enlarged choroid plexus with extensive edema around the ventricle, and this readily spreads to the ventricular wall (10). The MRI findings of our case that showed dense enhancement of the enlarged choroid plexus, ependymal spread and CSF dissemination with white matter edema along the ventricle were similar to those of choroid plexitis, and these findings showed rapid progression on the follow-up MRI. For that patient, the CSF examination revealed an increased WBC count with 59% lymphocytes, increased ADA and total protein, and a decreased glucose level, which were consistent with tuberculous CNS infection. Although germinoma may easily spread through the flow of the CSF or along the surfaces of the brain (5), it is hard for germinoma to be included in the differential diagnosis of our patient due to its location and rapid progression. These reasons led to a misdiagnosis of germinoma as choroid plexitis.
In summary, we experienced the first case of germinoma arising in the lateral ventricle. We also report that that intracranial germinoma can show rapid progression and it can mimic choroid plexitis by virtue of the rapid progression and the CSF profile.
Figures and Tables
Fig. 1
The axial postcontrast T1-weighted MR image (A and B) demonstrated the enlarged choroid plexus (arrow in figure A) and the adjacent ependymal enhancement in the trigone of the right lateral ventricle (arrows in figure B), and there is suspicious ependymal enhancement and a mildly enlarged choroid plexus in the left lateral ventricle. The axial FLAIR MR image (C) showed high signal intensities in the septum pellucidum (arrow in figure C) and along the trigone of the right lateral ventricle. The sagittal postcontrast T1-weighted MR image (D) demonstrated a suspicious mildly enlarged choroid plexus in the fourth ventricle, and no abnormal lesion in the pineal or suprasellar regions.
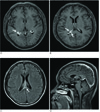
Fig. 2
The axial postcontrast T1-weighted MR images (A and B) demonstrate more progression of the dense enhancement of the enlarged choroid plexus (arrow in figure A) and adjacent ependymal enhancement in the right lateral ventricle and obvious dense enhancement of the enlarged choroid plexus (arrowhead in figure A) and ependymal enhancement (arrow in figure B) in the left ventricle. There are newly enhancing lesions in the ependyma of the right lateral ventricle. The axial FLAIR MR image (C) showed progression of the high signal intensities around both the lateral ventricles, and especially along the right lateral ventricle. The sagittal postcontrast T1-weighted MR image (D) depicts definite dense enhancement of the enlarged choroid plexus in the fourth ventricle (double arrows in figure D) and newly enhancing lesions have appeared in the pineal region (arrow in figure D) and the anterior wall of the fourth ventricle (arrowhead in figure D).
References
1. Tamaki N, Lin T, Shirataki K, Hosoda K, Kurata H, Matsumoto S, et al. Germ cell tumors of the thalamus and the basal ganglia. Childs Nerv Syst. 1990; 6:3–7.
2. Higano S, Takahashi S, Ishii K, Matsumoto K, Ikeda H, Sakamoto K. Germinoma originating in the basal ganglia and thalamus: MR and CT evaluation. AJNR Am J Neuroradiol. 1994; 15:1435–1441.
3. Sartori S, Laverda AM, Calderone M, Carollo C, Viscardi E, Faggin R, et al. Germinoma with synchronous involvement of midline and off-midline structures associated with progressive hemiparesis and hemiatrophy in a young adult. Childs Nerv Syst. 2007; 23:1341–1345.
4. Hoque R, Menon U, Gonzalez-Toledo E, Gu X, Jaffe SL. CNS germinoma of the pituitary and pineal regions, lateral ventricle, and fourth ventricle presenting in adulthood. J La State Med Soc. 2008; 160:319–321.
5. Smirniotopoulos JG, Rushing EJ, Mena H. Pineal region masses: differential diagnosis. Radiographics. 1992; 12:577–596.
6. Kon H, Kumabe T, Jokura H, Shirane R. Recurrent intracranial germinoma outside the initial radiation field with progressive malignant transformation. Acta Neurochir. 2002; 144:611–616.
7. Kobayashi T, Kageyama N, Kida Y, Yoshida J, Shibuya N, Okamura K. Unilateral germinomas involving the basal ganglia and thalamus. J Neurosurg. 1981; 55:55–62.
8. Tekkok IH, Sav A. Aggressive spinal germinoma with ascending metastases. J Neurooncol. 2005; 75:135–141.
9. Naeini RM, Yoo JH, Hunter JV. Spectrum of choroid plexus lesions in children. AJR Am J Roentgenol. 2009; 192:32–40.
10. Cho IC, Chang KH, Kim YH, Kim SH, Yu IK, Han MH. MRI features of choroid plexitis. Neuroradiology. 1998; 40:303–307.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download