Abstract
Secondary hemangiosarcomas are a rare malignancy that can develop in breast tissue that has undergone prior radiation therapy. Post-irradiation hemangiosarcomas (PIHs) have been frequently described in the literature, although, to the best of our knowledge, the imaging findings of locally recurred PIHs have not been previously reported. We present imaging findings of a case of a locally recurring secondary hemangiosarcoma in the context of prior breast conservation surgery and radiation.
Secondary breast hemangiosarcomas are a very rare malignancy and usually divided into two subtypes: lymphedema-associated cutaneous hemangiosarcomas and postirradiation hemangiosarcomas (PIHs) (12). PIHs are usually found in older women, who performed breast-conserving therapy and radiation therapy, several years after radiation therapy (1). According to previous reports, PIHs show a low incidence of 0.05-1.11% (2). However, the increasing use of breast-conserving therapy will predictably lead to an increased number of PIHs (3). In addition, the rate of local recurrence of PIHs is as high as 73%, and the five-year-survival rate is only 10-35% (4). Therefore, a high index of suspicion and an understanding of the imaging characteristics about this disease entity are necessary when dealing with patients with PIHs. Here we present mammographic and sonographic findings of a case of locally recurred secondary hemangiosarcoma in the context of prior breast-conservation therapy and radiation.
In January 2001, a 50-year-old woman underwent breast-conserving surgery with sentinel node biopsy of the ipsilateral axilla for a breast cancer in her right breast at our institution. The histologic report showed a 1.6 cm, well-differentiated infiltrating ductal carcinoma. The resection margins were free of tumor, but one lymph node had micrometastasis of less than 2 mm. The final TNM classification was T1N0M0. The patient underwent adjuvant external radiation therapy in the right breast with of 50.4 Gy plus a 9 Gy, as a boosting dose, for a total of 59.40 Gy over 50 days and five cycles of adjuvant chemotherapy with a cyclophosphamide and doxorubicin regimen. An annual follow-up mammography and biannual sonography revealed postoperative or radiation therapy-induced changes with no suspicious lesion until 2006.
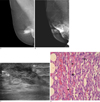
In December 2008, the patient complained of firmness and pain on the right periareolar region. On physical examination, a tense nipple with mild tenderness and ecchymosis of the surrounding skin was noted. Mammography showed progression of skin thickening and trabecular thickening of the right subareolar area, as compared with the findings from the previous mammography (Figs. 1A, B). On sonography, a 2.1 × 1.3 cm, irregular-shaped, angular marginated, and heterogeneous hypoechoic mass was found at the right subareolar area with extension to the thickened skin layer, assessed as Breast Imaging-Reporting and Data System (BI-RADS) category 4b (Fig. 1C). A core biopsy was performed immediately and revealed recurrent intraductal carcinoma. The patient underwent right-modified radical mastectomy (MRM). The final pathologic review revealed a hemangiosarcoma, which microscopically showed proliferation of spindle cells with nuclear atypia and a number of slit-like spaces containing RBCs (Fig. 1D).
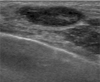
Four months later, a palpable nodule developed at the mastectomy site. On physical examination, 1.0 cm, well-demarcated nodules were palpated around the incision scar at the chest wall. On sonography, two ovalshaped, parallel, relatively circumscribed and hypoechoic nodules were detected around the incision scar (Fig. 2). A sonographically guided fine needle aspiration biopsy was performed for these lesions. Core biopsy could not be done because of patient's thin remnant chest wall and possibility of benign lesions. The final result of fine needle aspiration biopsy revealed a recurred hemangiosarcoma. The wide excision for these lesions has been considered.
Hemangiosarcomas are rare malignant tumors of the vascular endothelium. Approximately 8% of all hemangiosarcomas arise in the breast (4). Breast hemangiosarcomas are divided into primary and secondary subgroups (3). The mean age at presentation is the late 60s (3).
There are two types of secondary hemangiosarcoma; lymphedema-associated cutaneous hemangiosarcoma and postirradiation hemangiosarcoma (PIH) (1). A PIH usually affects the dermis of the breast within the radiation field (up to 85%), but may occasionally develop in the breast parenchyma (4). In our case, the mass was mainly located in the breast parenchyma and extended into the upper dermis. Although the underlying mechanism of the development of PIH is unknown, it has been assumed that radiation can act either directly via mutagenic effects on DNA or indirectly by causing lymph node sclerosis and, hence, lymphedema (23). According to previous reports, the incidence of PIH shows wide variation from 0.05 to 1.11% (2). The median interval between radiation therapy and development of a hemangiosarcoma was six years, and the median radiation dose was 50 Gy (2). Our case also showed a similar course, with a seven-year latency and 59.40 Gy radiation dose.
The clinical manifestation of PIH can present as overlying skin discoloration (purple or bluish), raised skin nodules, plaques, or a palpable mass, which can result in diagnostic delays due to misinterpretation as symptoms secondary to trauma or infection (13).
Several reports regarding the radiologic findings of breast PIH have been published. In a previous case report, the most common mammographic finding was progressive skin or trabecular thickening after several years of stability of post-radiation change (5). Another report evaluated sonographic findings in 24 patients with a breast hemangiosarcoma and concluded that hemangiosarcomas are primarily solitary masses with variable echo patterns-54% of the masses were hyperechoic or mixed hyper- and hypoechoic, whereas 46% of the masses were hypoechoic. This finding is different from breast carcinomas, which are rarely hyperechoic (6). Similarly, in our case, progression of skin and trabecular thickening was noticed on mammography and heterogeneous hypoechoic mass on sonography.
The initial effective therapy seems to be a mastectomy, including the wide excision of the margins and hyperfractionated radiation therapy or chemotherapy may be considered (24). In our case, the patient underwent MRM alone.
However, in spite of the complete resection of the mass, the local recurrence rate of PIH is as high as 73% (4). The overall survival is stated to be 72% after two years, with a 10-35% survival rate after five years (4). The median time to recurrence is six months (2). Possible poor prognostic factors of PIH include incomplete resection, multiple lesions, poorly differentiated or epithelioid lesions, accompanying lymphedema, and advancing age (23). One of the notable findings in our case is that the local recurrence of a hemangiosarcoma developed after a relatively short latency of four months from mastectomy, although the patient did not have any poor prognostic factors.
In addition, to our knowledge, there have been no discussions in the literature regarding the imaging characteristics of locally recurred secondary hemangiosarcomas. This case is worthy of notice in terms of different sonographic findings of the recurred lesion from primary lesion. The recurred lesions showed relatively circumscribed, hypoechoic, oval nodules, contrary to the primary lesion which appeared as an irregular-shaped, heterogeneous hypoechoic mass.
In conclusion, recurrence of a breast PIH may follow an unpredictable disease course and yield unexpected imaging findings. Therefore, high-grade suspicion and understanding about the breast PIHs is necessary when dealing with patients with prior radiation therapy on the breast.
Figures and Tables
Fig. 1
Imaging and histologic findings in a 57-year-old woman with breast PIH.
A. A mammographic image taken three years after radiation.
B. A mammographic image seven years after radiation shows progression of skin and trabecular thickening, compared with Fig. 1A.
C. A sonographic image shows a 2.1×1.3 cm, irregular-shaped, angular marginated, heterogeneous hypoechoic mass with extension to the thickened skin layer (arrows).
D. Microscopic findings of the mastectomy specimen show proliferation of the spindle cells with nuclear atypia (arrows) and a number of slit-like spaces containing RBCs (arrowheads), suggesting secondary hemangiosarcoma (H and E, ×400).
References
1. Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. AJR Am J Roentgenol. 2008; 190:533–538.
2. Abbott R, Palmieri C. Angiosarcoma of the breast following surgery and radiotherapy for breast cancer. Nat Clin Pract Oncol. 2008; 5:727–736.
3. West JG, Qureshi A, Wet JE, Chacon M, Sutherland ML, Haghighi B, et al. Risk of angiosarcoma following breast conservation: a clinical alert. Breast J. 2005; 11:115–123.
4. Kunkel T, Mylonas I, Mayr D, Friese K, Sommer HL. Recurrence of secondary angiosarcoma in a patient with post-radiated breast for breast cancer. Arch Gynecol Obstet. 2008; 278:497–501.
5. Moore A, Hendon A, Hester M, Samayoa L. Secondary Angiosarcoma of the breast: can imaging findings aid in the diagnosis? Breast J. 2008; 14:293–298.
6. Yang WT, Hennessy BT, Dryden MJ, Valero V, Hunt KK, Krishnamurthy S. Mammary angiosarcoma: imaging findings in 24 patients. Radiology. 2007; 242:725–734.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download