Abstract
Purpose
The purpose of this study is to develop image-based clinical decision support systems (CDSSs) using support vector machine models (SVMs) for the detection of seminal vesicle invasion (SVI) of prostate cancer and to compare the accuracies of 1.5T and 3.0T MR CDSSs.
Materials and Methods
A total of 548 prostate cancer patients who underwent a prostatectomy and preoperative MR using 1.5T or 3.0T were enrolled in this study. Each 1.5T and 3.0T group was subdivided into the training group and test group, arbitrarily. Images were analyzed in consensus by two radiologists. CDSS was constructed with input data that has the appearance of a seminal vesicle, PSA level and age in each training group, and with the output data of the probability for SVI using SVMs. The accuracy of the output data were evaluated with data of each test group. After a histopathologic correlation, the sensitivity, specificity and accuracy for the detection of SVI were compared in both 1.5T and 3.0T.
Figures and Tables
Fig. 1
Receiver operating characteristics (ROC) curve of clinical decision support systems using support vector machine (SVM) model at 1.5 T and 3.0T.
A. ROC curve of SVM model at 1.5 T machine shows the cut-off value 28.3 with sensitivity of 80.0% and specificity of 73.1%.
B. ROC curve of SVM model at 3 T machine shows the cut-off value of 31.8 with sensitivity of 72.7% and specificity of 90.4%.

Fig. 2
A 76-year-old man with PSA of 4.946 and high probability of seminal vesicle invasion suggested by 3.0T SVM model.
3.0T prostate MR axial (A) and coronal (B) images show wall destruction of seminal vesicle (arrows). The SVM model suggested that the probability of seminal vesicle invasion in this patient is 61.6. Regarding that the discriminating value in 3.0T SVM model is 31.8, the probability of seminal vesicle invasion in this patient is very high. The pathologic report revealed that seminal vesicle invasion is present.
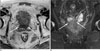
Fig. 3
A 77-year-old man with PSA of 15.3 and low probability of seminal vesicle invasion suggested by 1.5T SVM model.
1.5T prostate MR axial (A) and coronal (B) images show no definite abnormality in seminal vesicle. The SVM model suggested that the probability of seminal vesicle invasion in this patient is 27.6. Regarding that the discriminating value in 1.5T SVM model is 28.3, the probability of seminal vesicle invasion in this patient is low. However, the pathologic report revealed that seminal vesicle invasion is present.
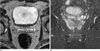
References
1. Won YJ, Sung JH, Jung KW, Kong HJ, Park SH, Shin HR, et al. Nationwide cancer incidence in Korea, 2003-2005. Cancer Res Treat. 2009; 41:122–131.
2. Kang T, Song CR, Song GH, Shin GH, Shin DI, Kim CS, et al. The anatomic distribution and pathological characteristics of prostate cancer: a mapping analysis. Korean J Urol. 2006; 47:578–585.
3. Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002; 167:528–534.
4. Koh H, Kattan MW, Scardino PT, Suyama K, Maru N, Slawin K, et al. A nomogram to predict seminal vesicle invasion by the extent and location of cancer in systematic biopsy results. J Urol. 2003; 170:1203–1208.
5. Sala E, Akin O, Moskowitz CS, Eisenberg HF, Kuroiwa K, Ishill NM, et al. Endorectal MR imaging in the evaluation of seminal vesicle invasion: diagnostic accuracy and multivariate feature analysis. Radiology. 2006; 238:929–937.
6. Jung DC, Lee HJ, Kim SH, Choe GY, Lee SE. Preoperative MR imaging in the evaluation of seminal vesicle invasion in prostate cancer: pattern analysis of seminal vesicle lesions. J Magn Reson Imaging. 2008; 28:144–150.
7. Ikonen S, Karkkainen P, Kivisaari L, Salo JO, Taari K, Vehmas T, et al. Endorectal magnetic resonance imaging of prostatic cancer: comparison between fat-suppressed T2-weighted fast spin echo and three-dimensional dual-echo, steady-state sequences. Eur Radiol. 2001; 11:236–241.
8. Ikonen S, Karkkainen P, Kivisaari L, Salo JO, Taari K, Vehmas T, et al. Magnetic resonance imaging of clinically localized prostatic cancer. J Urol. 1998; 159:915–919.
9. Rorvik J, Halvorsen OJ, Albrektsen G, Ersland L, Daehlin L, Haukaas S. MRI with an endorectal coil for staging of clinically localized prostate cancer prior to radical prostatectomy. Eur Radiol. 1999; 9:29–34.
10. Schiebler ML, Yankaskas BC, Tempany C, Spritzer CE, Rifkin MD, Pollack HM, et al. MR imaging in adenocarcinoma of the prostate: interobserver variation and efficacy for determining stage C disease. AJR Am J Roentgenol. 1992; 158:559–562.
11. Park BK, Kim BH, Kim CK, Lee HM, Kwon GY. Comparison of phased-array 3.0-T and endorectal 1.5-T magnetic resonance imaging in the evaluation of local staging accuracy for prostate cancer. J Comput Assist Tomogr. 2007; 31:534–538.
12. Sosna J, Pedrosa I, Dewolf WC, Mahallati H, Lenkinski RE, Rofsky NM. MR imaging of the prostate at 3 Tesla: comparison of an external phased-array coil to imaging with an endorectal coil at 1.5 Tesla. Acad Radiol. 2004; 11:857–886.
13. Torricelli P, Cinquantini F, Ligabue G, Bianchi G, Sighinolfi P, Romagnoli R. Comparative evaluation between external phased array coil at 3T and endorectal coil at 1.5T: preliminary results. J Comput Assist Tomogr. 2006; 30:355–336.
14. Suzuki H, Komiya A, Kamiya N, Imamoto T, Kawamura K, Miura J, et al. Development of a nomogram to predict probability of positive initial prostate biopsy among Japanese patients. Urology. 2006; 67:131–136.
15. Snow PB, Smith DS, Catalona WJ. Artificial neural networks in the diagnosis and prognosis of prostate cancer: a pilot study. J Urol. 1994; 152:1923–1926.
16. Stephan C, Cammann H, Semjonow A, Diamandis EP, Wymenga LF, Lein M, et al. Multicenter evaluation of an artificial neural network to increase the prostate cancer detection rate and reduce unnecessary biopsies. Clin Chem. 2002; 48:1279–1287.
17. Walz J, Graefen M, Chun FK, Erbersdobler A, Haese A, Steuber T, et al. High incidence of prostate cancer detected by saturation biopsy after previous negative biopsy series. Eur Urol. 2006; 50:498–505.
18. Nam RK, Toi A, Klotz LH, Trachtenberg J, Jewett MA, Appu S, et al. Assessing individual risk for prostate cancer. J Clin Oncol. 2007; 25:3582–3588.
19. Bianco FJ Jr. Nomograms and medicine. Eur Urol. 2006; 50:884–886.
20. Cortes C, Vapnik V. Support-vector networks. Mach Learn. 1995; 20:273–297.
21. Vapnik V. The nature of statistical learning theory. Berlin: Springer;2000. p. 123–160.
22. Comak E, Arslan A, Turkoglu I. A decision support system based on support vector machines for diagnosis of the heart valve diseases. Comput Biol Med. 2007; 37:21–27.
23. Blute ML, Bergstralh EJ, Iocca A, Scherer B, Zincke H. Use of Gleason score, prostate specific antigen, seminal vesicle and margin status to predict biochemical failure after radical prostatectomy. J Urol. 2001; 165:119–125.
24. Epstein JI, Partin AW, Potter SR, Walsh PC. Adenocarcinoma of the prostate invading the seminal vesicle: prognostic stratification based on pathologic parameters. Urology. 2000; 56:283–288.
25. Salomon L, Anastasiadis AG, Johnson CW, McKiernan JM, Goluboff ET, Abbou CC, et al. Seminal vesicle involvement after radical prostatectomy: predicting risk factors for progression. Urology. 2003; 62:304–309.
26. Vapnik V. Statistical learning theory, wiley series on adaptive and learning systems for signal processing, communications and control. New York: John Wiley & Sons;1998.
27. Duda RO, Peter EH, David GS. Pattern classification. 2nd ed. New York: Wiley-Interscience publication;2001.
28. Cristianini N, Shawe-Taylor J. An introduction to support vector machines and other kernel-based learning methods. Cambridge: Cambridge University Press;2000. p. 93–122.
29. Byvatov E, Fechner U, Sadowski J, Schneider G. Comparison of support vector machine and artificial neural network systems for drug/nondrug classification. J Chem Inf Comput Sci. 2003; 43:1882–1889.
30. Hearst M. Trends and controversies-support vector machines. IEEE Intell Syst. 1998; 13:18–28.
31. Beyersdorff D, Taymoorian K, Knosel T, Schnorr D, Felix R, Hamm B, et al. MRI of prostate cancer at 1.5 and 3.0T: comparison of image quality in tumor detection and staging. AJR Am J Roentgenol. 2005; 185:1214–1220.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download