Abstract
We report a case of progressive multifocal leukoencephalopathy (PML) isolated to the posterior fossa in a 55-year-old male with acquired immune deficiency syndrome(AIDS). Initial MR images revealed a few foci of patchy increased signal intensity(SI)on a T2-weighted image and a diffusion weighted image (DWI) at the pons, right middle cerebellar peduncle, and right cerebellar hemisphere, with no enhancement. After anti-retroviral therapy, follow-up MR images revealed the more prominent extent of previously-seen lesions and newly discovered newly developed focal increased SI on T2-weighted images located left of the inferior cerebellar hemisphere. Proton MR spectroscopy (1H-MRS) showed a slightly increased choline peak (3.2 ppm) and lactate peak (1.35 ppm), as well as a decreased N-acetylaspartate (NAA) peak (2.0 ppm), which suggests active demyelinating disease. DWI and 1H-MRS may support the diagnosis of PML in patients with AIDS.
Progressive multifocal leukoencephalopathy (PML) is a subacute demyelinating disease of the central nervous system (CNS) caused by neurotropic JC virus (JCV) and usually occurs in immunocompromised patients such as patients with acquired immunodeficiency syndrome (AIDS) (1). The prevalence of PML ranges from 4% to 7% of AIDS patients and has greatly increased over past years, corresponding to the rise in AIDS prevalence (2). This disease is usually progressive and the mean survival time is 6-9 months (3).
A brain biopsy is required to diagnose PML, but a noninvasive diagnosis of PML is needed in the early phase of its clinical course. Magnetic resonance imaging (MRI) is the most commonly used diagnostic method to evaluate patients with AIDS who have neurologic symptoms and access to treatment response (45). Aside from conventional MRI, diffusion weighted imaging (DWI), and proton MR spectroscopy (1H-MRS) were used to support the diagnosis made during these days.
In this report, we demonstrate the characteristic features of DWI and 1H-MRS in a patient with AIDS-related PML. Conventional initial and follow-up MRI findings are also presented and described in connection with the poor prognosis of PML.
A 55-year-old man with no significant past medical history had a 3-week history of worsening dysarthria and right arm weakness. Upon physical examination, he was afebrile with normal vital signs. A neurologic examination revealed dysarthria, right upper extremity weakness (grade IV), and right side ataxia. A microscopic analysis revealed no bacterial or fungal elements and a cerebral spinal fluid (CSF) study was negative for gram stain, india ink stain, and acid fast bacilli (AFB) stain. However, CSF polymerase chain reaction (PCR) corresponding to JCV deoxyribonucleic acid (DNA) was positive. A human immunodeficiency virus (HIV)-1 antibody test was also positive. The CD4+ T-lymphocyte count was determined to be 189/µl (normal range, 500-1,200 µl). However, a brain biopsy could not be performed due to patient refusal a worsening clinical state.
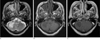
MRI, including DWI and apparent diffusion coefficient (ADC) mapping was obtained with a 1.5T (Siemens 1.5 T, Sonata, Germany) scanner. Initial MRI showed a few foci of patchy increased signal intensity (SI) on T2-weighted images (WI) at the pons, right middle cerebellar peduncle, and right cerebellar hemisphere, with no enhancement (Fig. 1). An initial DWI revealed high SI at the right middle cerebellar peduncle and peripheral marginal zone of the right cerebellar hemisphere. ADC maps showed high SI at the right middle cerebellar peduncle and center of the right cerebellar hemisphere, but low to normal SI at the peripheral marginal zone of the right cerebellar hemisphere (Fig. 2). Deep gray matter structures were spared on all MR images. 1H-MRS revealed a slight increase in the choline peak (3.2 ppm) and lactate peak (1.35 ppm), as well as a decreased N-acetylaspartate (NAA) peak (2.0 ppm), suggesting active demyelinating disease (Fig. 3). Despite the highly active antiretroviral therapy (HAART), the patient's clinical course worsened with the development of diplopia and gait disturbance, as well as progressive ataxia. At hospital day 22, a follow-up MRI showed a slight extension of previous lesions (Fig. 4) as well as a newly developed focal increased SI on T2-WI at the left inferior cerebellar hemisphere. Six weeks after the onset of antiretroviral therapy, the patient developed general fever, disturbance of consciousness, and acute respiratory distress syndrome (ARDS) and expired three days later.
The PML associated with AIDS has a poor prognosis and survival time is usually less than 9 months (3). Although recent studies have reported patients with AIDS-related PML, which were improved by HAART (4), our patient had no effective response for the combination of anti-retroviral agents.
In PML patients, the differential diagnosis from mimicking disease such as ischemic stroke, multiple sclerosis, or MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and a stroke-like episode) can benefit from DWI and ADC mapping (5). Characteristic DWI and ADC mapping features of PML lesions have high SI on DWI and normal-to-low SI on ADC maps at the peripheral margin, while have low SI on DWI and high SI on ADC maps at the center. In our patient, we also found the similar features of DWI and ADC maps at the peripheral margin and center of the right cerebellar hemisphere (Fig. 2). These distinctive features will be able to support the differential diagnosis from the foregoing mimicking diseases. We suggest that these DWI and ADC features of PML-related lesions could be called a "layered phenomenon".
1H-MRS for patients with PML usually show an increase in lactate/Cr (creatine), Cho/Cr, and lipids/Cr ratios as well as a decrease in NAA/Cr ratio, compared to the normal contralateral region (6). The 1H-MRS for our case also revealed an increased choline peak (3.2 ppm) and lactate peak (1.35 ppm), as well as a a decreased NAA peak (2.0 ppm) (Fig. 3). These findings suggest that PML is demyelinating disease, therefore, 1H-MRS can be used to support the diagnosis of PML in patients with AIDS.
Conventional MRI of AIDS-related PML usually reveals asymmetrically increased SI on T2-WI and decreased SI on T1-WI at white matter (most commonly parietal white matter). The involvement of another brain region such as the cerebellum, brainstem, or cortical gray matter is rare but does happen. Post et al. (7) reported the only three of 48 patients with AIDS related PML had disease isolated to posterior fossa. In our case, the involvement of the brain region only included the cerebellum without a supratentorial white matter lesion (Fig. 1). Until now, few case reports of AIDS-related PML were isolated to the cerebellum.
Typical PML lesions usually have no contrast enhancement. However, faint and marginal contrast enhancing PML lesions have been described on a few studies (48). Until now, the contrast enhancement of PML lesions has been thought to be a result of intense inflammatory reaction and one of the predictive factors for prolonged survival (49). Thuruher et al. (4) suggested that the enhancement of PML lesions isa feature of patients who experienced immunological reconstitution in the early phase of anti-retroviral therapy. Our patient had a low CD4+ count at the time of diagnosis and over the course of anti-retroviral therapy, while the CD4+ count did not rise. No contrast enhancement of PML lesion was observed on the initial MRI (Fig. 1) and follow-up MRI (Fig. 4). Ultimately the patient had a poor prognosis, and finally died. As a result, we agree with the previous findings for the contrast enhancement of PML lesions, which suggest that a PML lesion with no contrast enhancement is related with poor prognosis.
There have been a few published reports examining whether there was a correlation between mass effect and PML as well as survival time. Post et al. (7) suggested that the presence of mass effect correlated with shorter survival time, while Thurnher et al. (4) mentioned the development of mass effect in the phase immediately following therapy might have resulted from transient edema rather than the progression of the PML. In an initial and follow-up MRI of our patient, there was no mass effect associated with the PML lesion (Figs. 1, 2, 3, 4). Based on our case, we think that the absence of mass effect is not necessarily correlated with longer survival time. The relationship between mass effect of the PML lesion and the patient's prognosis is an area that needs more research.
Figures and Tables
Fig. 1
Initial MRI (A: T2-weighted image (WI), B: T1-WI, C: contrast enhanced T1-WI) revealed a few foci with patchy increased signal intensity (SI) on T2-WI and decreased SI on T1-WI at the pons, right middle cerebellar peduncle, and right cerebellar hemisphere with no enhancement.

Fig. 2
DWI (A) and ADC map (B) showed high signal intensity (SI) on DWI and normal-to-low SI on an ADC map at the peripheral margin of right cerebellar hemisphere, while low SI on DWI and high SI on an ADC map at the center. We suggest that these characteristic features of DWI and the ADC map could be called a "layered phenomenon".

Fig. 3
MR spectroscopy showed a slightly increased choline peak (3.2 ppm) and lactate peak (1.35 ppm), as well as a decreased N-acetylaspartate (NAA) peak (2.0 ppm), suggesting demyelinating disease.

Fig. 4
Twenty-two day follow-up MRI (A: T2-weighted image (WI), B: T1-WI, C: contrast enhanced T1-WI) showed a slight extension of the previous lesions at the pons, right middle cerebellar peduncle, and right cerebellar hemisphere with newly developed (not seen) focal high SI on T2-WI at the left inferior cerebellar hemisphere.
References
1. Brooks BR, Walker DL. Progressive multifocal leukoencephalopathy. Neurol Clin. 1984; 2:299–313.
2. Holman RC, Janssen RS, Buehler JW, Zelasky MT, Hooper WC. Epidermiology of progressive multifocal leukoencephalopathy in the United States: analysis of national mortality and AIDS surveillance data. Neurology. 1991; 41:1733–1736.
3. Berger JR, Kaszovitz B, Post MJ, Dickinson G. Progressive multifocal leucoencephalopathy associated with human immunodeficiency virus: a review of the literature with a report of sixteen cases. Ann Intern Med. 1987; 107:78–87.
4. Thurnher MM, Post MJ, Rieger A, Kleible-Popov C, Loewe C, Schindker E. Initial and follow-up MR imaging findings in AIDS related progressive multifocal leukoencephalopathy treated with highly active antiretroviral therapy. AJNR Am J Neuroradiol. 2001; 22:977–984.
5. Yoon JH, Bang OY, Kim HS. Progressive multifocal leucoencephalopathy in AIDS: proton MR spectroscopy patterns of asynchronous lesions confirmed by serial diffusion-weighted imaging and apparent diffusion coefficient mapping. J Clin Neurol. 2007; 3:200–203.
6. Chang L, Ernst T, Tornatore C, Aronow H, Melchor R, Walot I, et al. Metabolite abnormalities in progressive multifocal leukoencephalopathy by proton magnetic resonance spectroscopy. Neurology. 1997; 48:836–845.
7. Post MJ, Yiannoutsos C, Simpson D, Booss J, Clifford DB, Cohen B, et al. Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? AIDS Clinical Trials Group, 243 Team. AJNR Am J Neuroradiol. 1999; 20:1896–1906.
8. Whiteman ML, Post MJ, Berger JR, Tate LG, Bell MD, Limonte LP. Progressive multifocal leukoencephalopathy in 47 HIV-seropositive patients: neuroimaging with clinical and pathologic correlation. Radiology. 1993; 187:233–240.
9. Kotecha N, George MJ, Smith TW, Corvi F, Litofsky NS. Enhancing progressive multifocal leukoencephalopathy: an indicator of improved immune status. Am J Med. 1998; 105:541–543.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download