Abstract
Materials and Methods
For 3 years from 2006, 180 patients (mean age 48-years-old) with 187 breast cancers underwent positron emission tomography-computed tomography (PET/CT; biograph2, Siemens) at our institute and were enrolled in this study. We evaluated whether there was a correlation between the peak standardized uptake value (pSUV) of PET/CT and the histologic type of the breast cancers (n=187), grade of the invasive ductal cancers (n=142), and tumor size (n=153).
Results
The different histologic types of breast cancers include IDCs (n=156), in situ ductal carcinoma (n=10), papillary cancer (n=6), mucinous cancer (n=6), invasive lobular cancer (n=4), medullary cancer (n=3), metaplastic cancer (n=1), and neuroendocrine cancer (n=1). pSUV showed significant differences according to histologic type (p<0.005). For the available cases (n=142), IDCs were classified as grade 1 (n=25), grade 2 (n=66), and grade 3 (n=51) and correlated with the histologic grade of IDCs (rho=0.41, p<0.001). pSUV was correlated with tumor size regardless of histologic type (rho=0.525, p<0.001). In low grade IDCs, pSUV was correlated with tumor size (rho=0.48-0.86, p<0.001), but not in high grade IDCs (p>0.001).
Figures and Tables
Fig. 2
pSUV of IDCs according to histologic grading.
A-C. Within IDCs, pSUV was significantly correlated with histologic grading. (A) PET/CT image of 44-year-old woman having IDC grade 1 in upper inner quadrant of left breast and performed left mastectomy. pSUV of cancer was 2.6 and the histologic size was 2 cm. (B) PET/CT image of 28-year-old woman having IDC grade 2 in upper outer quadrant of left breast and performed breast conserving surgery. pSUV of cancer was 5.0 and the histologic size was 2 cm. (C) PET/CT image of 56-year-old woman having IDC grade 3 in upper outer quadrant of right breast and performed left mastectomy. pSUV of cancer was 10.9 and the histologic size was 2 cm.
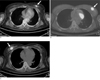
Fig. 3
Distribution of breast cancers according to size of tumor and pSUV.
Regardless of histology, pSUV was correlated with tumor size (rho=0.525, p<0.001).
Note: pSUV=peak standardized uptake value, IDC=invasive ductal carcinoma, DCIS=ductal carcinoma in situ, NDIC=non-ductal invasive cancer

References
1. Oh GG, Moon WG, Son EJ, Kim MH, Jung SY, Ko GR, et al. 유방질환의 영상검사. In : Jung SY, Han BG, editors. Korean Society of Breast Imaging. Breast Diagnostic Imaging. Ilchokak;2008. p. 121.
2. Rosen EL, Eubank WB, Mankoff DA. FDG PET, PET/CT, and
breast cancer imaging. Radiographics. 2007; 27:S215–S229.
3. Lim HS, Yoon W, Chung TW, Kim JK, Park JG, Kang HK, et al. FDG PET/CT for the detection and evaluation of breast diseases: usefulness and limitations. Radiographics. 2007; 27:S197–S213.
4. Noh DY, Yun IJ, Kim JS, Kang HS, Lee DS, Chung JK, et al. Diagnostic value of positron emission tomography for detecting breast cancer. World J Surg. 1998; 22:223–227.
5. Wahl RL, Cody RL, Hutchins GD, Mudgett EE. Primary and metastatic breast carcinoma: initial clinical evaluation with PET with the radiolabeled glucose analogue 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1991; 179:765–770.
6. Crowe JP, Adler LP, Shenk RR, Sunshine J. Positron emission tomography and breast masses comparison with clinical, mammographic, and pathological findings. Ann Surg Oncol. 1994; 1:132–140.
7. Moon DH, Maddahi J, Silverman DH, Glaspy JA, Phelps ME, Hoh CK. Accuracy of whole-body fluorine-18-FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med. 1998; 39:431–435.
8. Palmedo H, Bender H, Grunwald F, Mallmann P, Zamora P, Krebs D, et al. Comparison of fluorine-18 fluorodeoxyglucose positron emission tomography and technetium-99 m methoxyisobuty lisonitrile scintimammography in the detection of breast tumours. Eur J Nucl Med. 1997; 24:1138–1145.
9. Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M, Alavi A. Clinicopathologic factors associated with false negative FDG-PET in primary breast cancer. Breast Cancer Res Treat. 2006; 98:267–274.
10. Avril N, Rose CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000; 18:3495–3502.
11. Samson DJ, Flamm CR, Pisano ED, Aronson N. Should FDG PET be used to decide whether a patient with an abnormal mammogram or breast finding at physical examination should undergo biopsy? Acad Radiol. 2002; 9:773–783.
12. Adler LP, Crowe JP, al-Kaisi NK, Sunshine JL. evaluation of breast masses and axillary lymph nodes with [F-18] 2-deoxy-2- fluoro-D-glucose PET. Radiology. 1993; 187:743–750.
13. Schirrmeister H, Kuhn T, Guhlmann A, Santjohanser C, Horster T, Nussel K, et al. Fluorine-182-deoxy-2-fluoro-D-glucose PET in the preoperative staging of breast cancer: comparison with the standard staging procedures. Eur J Nucl Med. 2001; 28:351–358.
14. Kumar R, Alavi A. Fluorodeoxyglucose-PET in the management of breast cancer. Radiol Clin North Am. 2004; 42:1113–1122.
15. Eubank WB, Mankoff DA. Evolving role of positron emission tomography in breast cancer imaging. Semin Nucl Med. 2005; 35:84–99.
16. Crippa F, Seregni E, Agresti R, Chiesa C, Pascali C, Bogni A, et al. Association between [18F]fluorodeoxyglucose uptake and postoperative histopathology, hormone receptor status, thymidine labelling index and p53 in primary breast cancer: a preliminary observation. Eur J Nucl Med. 1998; 25:1429–1434.
17. Avril N, Menzel M, Dose J, Schelling M, Weber W, Janicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001; 42:9–16.
18. Buck A, Schirrmeister H, Kuhn T, Shen C, Kalker T, Kotzerke J, et al. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging. 2002; 29:1317–1323.
19. Buck AK, Schirrmeister H, Mattfeldt T, Reske SN. Biological characterisation of breast cancer by means of PET. Eur J Nucl Med Mol Imaging. 2004; 31:Suppl 1. S80–S87.
20. Yoo JH, Choi HY, Ahn SH. Misdignosed Breast Cancer on Mammography: Retrospectie Analysis in 17 Cases. J Korean Radiol Soc. 1995; 32:501–506.
21. Kim YC, Park HJ, Han H, Lim DM, Chung HS, Kim JE, Kim YC. Analysis of Mammographic Findings of Breast Cancer. J Korean Radiol Soc. 1995; 32:337–342.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download