Abstract
Inflammatory myofibroblastic tumor (IMT) is a rare condition of unknown origin. Pathologically, the lesion is composed of myofibroblastic spindle cells accompanied by an inflammatory infiltrate of plasma cells, lymphocytes, and eosinophils. We report two cases of inflammatory myofibroblastic tumor of the bladder which showed different imaging features and was falsely diagnosed as malignant tumors. We discuss the imaging findings along with a literature review.
Inflammatory myofibroblastic tumor (IMT) is a rare but distinctive lesion with uncertain malignant potential and unknown etiology. IMT has been reported in various anatomic sites, but is rare in genitourinary tract. Pathologically, the lesion is composed of dominant spindle cell proliferation with variable amounts of an inflammatory component. These spindle cells are now known to be myofibroblasts, which account for the current designation for this disease (1234). IMT presents a diagnostic dilemma for radiologists and pathologists because the radiologic and histological features often mimic those of a malignant neoplasm. However, the clinical course is known to be benign as the tumor grows slowly and does not metastasize or undergo a malignant transformation (2).
A 26-year-old woman presented with gross hematuria. She had a history of cystitis over the course of the last month. A physical examination revealed tenderness in the suprapubic region. A urinalysis showed gross hematuria and microscopic pyuria and a cytological analysis of the urine failed to show malignant cells. Laboratory studies were normal except for mild leukocystosis. Cystoscopy showed a broad based protruding mass at the anterosuperior wall of bladder. The mucosal surface appeared to be intact, but the evaluation was limited due to a large amount of hematoma and scattered blood clots covering the mass. CT showed a well enhancing mass at the bladder dome (Figs. 1A-C). The right side of the mass showed relative homogeneous enhancement, whereas the left portion of the mass was not enhanced, which indicated necrosis. A low attenuation filling defect was seen in the dependent portion of the bladder lumen, which indicated hematoma. Ultrasonogra-phy was performed and revealed a heterogeneous hypoechoic mass at the bladder dome. Color Doppler images showed prominent vascular flow with arterial pulse on the right side of the mass. A transurethral resection was performed and a biopsy of the mass showed inflammatory myxoid tumor, which should be differentiated from infiltrating urotherlial carcinoma with sarcomatoid variant. A laparoscopic partial cystectomy was performed and the gross specimen showed a large irregular protruding mass. Microscopically, the tumor was composed of spindle cells intermixed with an inflammatory component (Fig. 1D). Though a portion of the mucosal surface was intact, multifocal mucosal destruction with hemorrhage and ulceration was noted. Immunohistochemical staining demonstrated that spindle cells were positive for vimentin, p53, and cytokeratin, while they were negative for anaplastic lymphoma kinase-1 (ALK-1) and IgG4. The histomorphology and immunophenotype of the mass and overall clinical courses were considered for the final diagnosis of IMT.
A 51-year-old woman transferred from an outside hospital to properly manage a known bladder mass. On three separate occasions, she underwent a transurethral resection of the bladder tumor due to recurrent transitional cell carcinoma from 2004 to 2006. A contrast-enhanced CT scan showed polypoid enhancing mass at the left lateral aspect of the bladder (Figs. 2A-C). A transurethral resection of bladder tumor was performed, which revealed multiple and sessile masses located at the lateral wall of the bladder. Microscopically the tumor was composed of bland spindle cells in fibrous stroma with a prominently plasmacystic infiltrate (Fig. 2D). Immunohistochemically, the tumor cells stained strongly for vimentine and focally for desmin, but were negative for cytokeratin, actin, IgG4 and ALK-1. The pathologic diagnosis was consistent with an inflammatory myofibroblastic tumor.
Inflammatory myofibroblastic tumors are also known as an inflammatory pseudotumor, atypical fibroblastic tumor, atypical fibromyxoid tumor, and plasma cell granuloma (5). These tumors most commonly occur in the urinary bladder among the genitourinary tract, and typically appear in young adults although IMTs rarely occur in the genitourinary tract (356). Age predilection is however a controversial issue (1267). The causes of this lesion are not well understood, but it has been suspected to be a result from inflammation following trauma, surgery, or infection, since the pathologic findings of the inflammatory myofibroblastic tumor show spindle cells identical with myofibroblasts shown at granulomatous tissue (145).
Although IMT may be found at any site on the bladder, according to a previous report, the involvement site of IMT has a predilection at the superior wall of bladder (8). Our case 1 also showed a similar location of involvement. These masses tend to spare the trigone (156); however, large lesions may invade through the bladder wall and may have a substantial extravesical component, making differentiation from a malignant process impossible (1).
Inflammatory myofibroblastic tumors appear as a single polypoid intraluminal or submucosal mass, which may be ulcerated or show central necrosis (1257). IMTs may show strong enhancement on the contrastenhanced imaging study (56) and also show the rim or ring enhancement (17). On color Doppler sonography, these lesions may show hypervascularity (5). Our two cases showed different imaging features. Case 1 appeared as a large smooth-surfaced mass with hypervascularity and hemorrhage similar to previous reported cases, which was not consistent common urothelial carcinoma of bladder. Our preoperative differential diagnoses were sarcoma or urachal remnant carcinoma due to its aggressive nature, location, and the patient's age. Case 2 showed a nodular and infiltrating pattern associated with bladder wall thickening, which mimicked urothelial carcinoma of bladder. We also falsely diagnosed case 2 as recurrent urothelial carcinoma as a result of taking into consideration the past history of the patient and imaging features. When considering the surgical history of transurethral resection in case 2, a postoperative spindle cell nodule should be included in the differential diagnosis. The microscopic findings of the postoperative spindle cell nodule are fascicles of spindle cells mixed with inflammatory cells, which are very similar to IMT. The diagnostic clues for postoperative spindle cell nodule are postoperative mass formation, usually within less than 3 months after a previous surgical procedure at the surgical resection site (9). For our case 2, the mass developed 2 years after previous surgery. However, the location of previous operation site was not known due to prior imaging studies not being available.
Pathologically, because of its cytologic features and infiltrative nature, it may be difficult to distinguish from sarcomatous proliferations (13). Recently, anaplastic lymphoma kinase (ALK) gene translocations or ALK protein expression in inflammatory myofibroblastic pseudotumor, has been reported, especially in patients of a relatively young age (6). However, the differentiation from carcinoma with sarcomatoid differentiation was difficult in our case 1 because it was negative for ALK-1 and strong positive for cytokeratin; the latter condition is a marker of carcinoma. The final pathologic diagnosis was concluded to be an inflammatory myofibroblastic tumor with regard to combined clinical, gross, microscopic pathologic features, and other immunohistochemical stains such as Vimentin and p53.
Some recent pathologic reports say that IMT should be differentiated from non-specific inflammatory pseudotumors, and the differentiation between two disease entities could be assisted by IgG4 (10). According to this report, IMT is not immunoreactive for IgG4, while inflammatory pseudotumors are immunoreactive for IgG4. Both of our two cases were not immunoreactive for IgG4.
Our two cases were difficult to diagnose with preoperative imaging findings, although these cases have predisposing factors such as recurrent cystitis and previous bladder surgery. IMT should be included in the differential diagnosis of a hypervascular bladder mass, especially in patients with predisposing factors for IMT. However, further radiologic and pathologic studies for IMTs showing different imaging features will be required.
Figures and Tables
Fig. 1
A 28-year-old woman presented with inflammatory myofibroblastic tumor. Color Doppler sonogram (A) shows a hypoechoic and hypervascular mass in the anterior wall of the bladder dome. Contrast-enhanced axial (B) and sagittal (C) CT scans show a well contrast enhancing portion (long arrow in B and C) on the right side of the mass. Moreover, a poorly enhancing area (asterisk) was evident in the left posterior portion. An irregular-shaped lesion (open arrow in B) was seen in the dependent portion of the contrast-filled bladder, suggesting hematoma. Microphotography (D) shows a proliferation of atypical spindle cells (arrows) and inflammatory cell infiltration in a myxoid background.
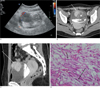
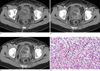
Fig. 2
A 51-year-old woman with an inflammatory myofibroblastic tumor. Dynamic contrast-enhanced axial CT scans show a rounded mass (arrows in A-C) with strong enhancement on arterial (B) and portal (C) phase, arising from the left lateral aspect of the bladder. Note the diffuse and irregular bladder wall thickening. Microphotography (D) shows a proliferation of atypical spindle cells (arrows) and inflammatory cell infiltration with a myxoid background.
References
1. Wong-You-Cheong JJ, Woodward PJ, Manning MA, Davis CJ. Inflammatory and non-neoplastic bladder masses: radiologicpathologic correlation. Radiographics. 2006; 26:1847–1868.
2. Kumar A, Bhatti SS, Sharma S, Gupta SD, Kumar R. Inflammatory pseudotumor of urinary bladder: a diagnostic and management dilemma. Int Urol Nephrol. 2007; 39:799–802.
3. Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003; 23:719–729.
4. Mochizuki Y, Kanda S, Nomata K, Hayashi T, Yamasaki Y, Kanetake H, et al. Spontaneous regression of inflammatory pseudotumor of the urinary bladder. Urol Int. 1999; 63:255–257.
5. Kim SH, Cho JY, Kim SH. Inflammatory myofibroblastic pseudotumor of the urinary bladder in a patient with bilateral renal cell carcinoma. J Ultrasound Med. 2008; 27:483–486.
6. Park SB, Cho KS, Kim JK, Lee JH, Jeoung AK, Kwon WJ, et al. Inflammatory pseudotumor (myoblastic tumor) of genitourinary tract. AJR Am J Roentgenol. 2008; 191:1255–1262.
7. Houben CH, Chan A, Lee KH, Tam YH, To KF, Cheung W, et al. Inflammatory myofibroblastic tumor of the bladder in children: what can be expected. Pediatr Surg Int. 2007; 23:815–819.
8. Sugita R, Saito M, Miura M, Yuda F. Inflammatory pseudotumour of the bladder: CT and MRI findings. Br J Radiol. 1999; 72:809–811.
9. Young RH. Tumor-like lesions of the urinary bladder. Mod Pathol. 2009; 22:Suppl 2. S37–S52.
10. Ymamoto H, Yamaguchi H, Aishima S, Oda Y, Kohashi K, Oshiro Y, et al. Inflammatory myofibroblastic tumor versus IgG4-related sclerosing disease and inflammatory pseudotumor: a comparative clinicopathologic study. Am J Surg Pathol. 2009; 33:1330–1340.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download