Abstract
Primary pancreatic lymphoma is extremely rare and usually consists of a bulky tumor without internal necrosis and severe dilatation of the pancreatic duct. We report the unusual CT and MRI findings of a primary pancreatic lymphoma in a 27-years-old man, associated with severe dilatation of the pancreatic duct, common bile duct, and internal necrosis.
Primary pancreatic lymphoma (PPL) is a rare extralymphatic lymphoma. It is usually found as a large pancreatic mass with no dilatation of the pancreatic duct. Severe dilatations of the pancreatic duct and common bile duct in PPL have not been described. Moreover, internal necrosis in PPL is extremely rare in the literature. Here, we report CT and MRI findings of PPL with severe dilatation of the pancreatic duct, as well as internal necrosis in a 27-year-old man.
A 27-year-old man presented with a history of fatigue, jaundice, and weight loss for 20 days. His past medical history revealed that he was a hepatitis B carrier. However, his physical examination was normal without evidence of abdominal tenderness or masses. His serum total albumin, direct albumin, aspartate amino transferase (AST), and alanine amino transferase (ALT) were elevated; however, his serum amylase and lipase levels were normal, as were other laboratory tests including leukocyte counts. Contrast enhanced multi-detector row CT (MDCT) showed a 5 cm long diameter and poorly enhancing mass containing a low attenuated area in the pancreas head, with moderate to severe dilatation of pancreatic duct, common bile duct, and both intrahepatic bile ducts (Fig. 1A). Lymphadenopathy was not demonstrated; however, displacement of portal vein, IVC, and duodenum was depicted. Abdominal MR imaging was performed for the purpose of planning surgery on a 3T MRI unit using a phased array coil. T1-weighted MR images showed a hypointense homogeneous mass with an internal hyperintense area (Fig. 1B). T2-weighted MR images demonstrated a hyperintense mass with a hyperintense area, as well as severe dilatation of the pancreatic duct (Fig. 1C). A hyperintense area on T1- and T2-weighted MR images was considered as hemorrhage within the mass. However, gadolinium-enhanced T1-weighted MR images showed homogeneous enhancement (Fig. 1D). ERCP demonstrated severe dilatation of the distal pancreatic duct, both intrahepatic bile ducts, and the common bile duct (Fig. 1E). The preoperative diagnosis was a neuroendocrine tumor or pancreatic ductal adenocarcinoma due to severe dilatation of the pancreatic duct and common bile duct as well as the internal heterogeneous area. An endoscopic US-guided biopsy was performed, but failed. We decided to perform surgery because of the patient's young age and high probability of malignancy on the imaging study. A pylorus preserving pancreaticoduodenectomy was performed, without preoperative chemotherapy.
Upon gross inspection of the surgical specimen, a well circumscribed whitish mass was identified with the narrowing of the downstream pancreatic duct and common bile duct. The tumor invaded the pancreatic duct and duodenal wall (Fig. 2A). A microscopic examination showed focal, well-circumscribed coagulative necrosis within the tumor, considered as hemorrhage on a preoperative MRI (Fig. 2B). The tumor cells displayed a large, round to oval shaped nucleus, scanty cytoplasm, and prominent nucleoli. Immunostaining results indicated that the tumor cells showed positive immunoreactivity for CD20 and CD79a, and negative immunoreactivity for cytokeratin, CD45RO, and CD3 (Fig. 2C). The tumor was pathologically confirmed as malignant diffuse large B-cell lymphoma. The patient had received postoperative adjuvant CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy and has been living without evidence of tumor recurrence over 17 months after surgery.
PPL is an extremely rare disease which occurs in pancreatic situ, with or without involvement of the peripancreatic lymph nodes (1). The diagnostic criteria for PPL include: 1) no palpable superficial lymphadenopathy, no mediastinal lymphadenopathy on chest radiography, 2) a normal leukocyte count in peripheral blood, 3) a main mass in the pancreas with lymph nodal involvement confined to the peripancreatic region, and 4) no hepatic or splenic involvement (2). Most cases are actually primary low-grade non-Hodgkin's lymphoma of the B-cell type. Our case was pathologically confirmed as malignant diffuse large B-cell lymphoma.
On CT, there are two different morphologic patterns of pancreatic involvement in patients with PPL. The former is a localized, well circumscribed tumoral form, and the latter is a diffusely enlarged infiltrating or replacing form occupying most of the pancreatic gland (3). In a well circumscribed tumoral type, an MRI shows a homogeneous hypointense mass within the pancreas on T1-weighted images with subtle enhancement after gadolinium enhancement, as well as a heterogeneous mass with low to intermediate signal intensity on T2-weighted images. As for a diffuse infiltrating tumor type, T1- and T2-weighted images showed hypointense enlarged pancreas, with mild to moderate enhancement after gadolinium enhancement (4). In our case, the mass was also homogeneous on CT and MRI with mild homogeneous enhancement, but the mass contained a necrotic area as a hypoattenuation on CT and a hyperintense area on T1- and T2-weighted MR images.
Internal hypoattenuation on CT and a hyperintense area on T1- and T2-weighted MR images were considered as hemorrhage within the mass. The presences of calcification or necrosis are reliable findings for ruling out non-Hodgkin's lymphoma (5). However, a small heterogeneous area which was necrosis within a tumor can be seen in an isolated case (6). In our case, focal necrosis within the mass was shown on CT and MRI. This finding was very unusual in untreated non-Hodgkin's lymphoma and made the diagnosis of PPL difficult.
Unlike pancreatic ductal adenocarcinoma, moderate to severe dilatation of the pancreatic duct is apparently extremely rare in PPL because the pancreatic duct is either normal, displaced, or simply narrowed in patients with PPL (4). Van Beers B et al. (3) reported that dilatation of the pancreatic duct can be usually mild, with a ratio of duct diameter to distal gland width invariably less than 0.5 in PPL. Severe dilatation of the pancreatic duct and common bile duct in our case was demonstrated on CT and MRI. A dilated pancreatic duct and common bile duct on CT and MRI led us to preoperatively diagnose a neuroendocrine tumor or pancreatic ductal adenocarcinoma.
During the surgical procedure, the hard mass with internal necrosis was seen at the pancreatic head along with moderate to severe dilatation of common bile duct and pancreatic duct. The hardness of the tumor and the presence of internal necrosis suggested a rapid growth rate and were considered as cause of the dilatation of the common bile duct and pancreatic duct, which was different to usual pancreatic lymphoma.
The differential diagnosis of localized PPL in our case included pancreatic ductal adenocarcinoma and neuroendocrine tumors such as a large islet cell tumor. The differential diagnosis of PPL from the pancreatic ductal adenocarcinoma is very important. For the pancreatic ductal adenocarcinoma, the primary treatment is considered to be surgical excision; but in PPL, the primary treatment is nonsurgical, based on chemotherapy alone or a combination of chemotherapy and radiation therapy (1). Ductal adenocarcinoma commonly infiltrates into pancreatic parenchyma, peripancreatic fat, as well as adjacent structures, and dilates the more distal pancreatic duct when the more proximal ductal invasion has taken place. Also CT findings of ductal adenocarcinoma typically manifest themselves as a lesion that is somewhat inhomogeneous and hypoattenuating relative to the normally enhancing pancreatic parenchyma. Neuroendocrine tumors such as the large non-functioning islet cell tumor demonstrate hypervascular solid components, usually in the periphery, and central non-enhancing areas on CT, which may represent necrosis, fibrosis, or cystic degeneration (7). Also, islet cell tumors usually show early strong enhancement on early-dynamic contrast CT or MRI as well as frequent hepatic metastasis (8). However PPL usually demonstrates poor but homogeneous enhancement (9).
In summary, we present a rare case of PPL with unusual imaging findings. Although a bulky homogeneous mass with moderate to severe dilatation of the pancreatic duct and focal necrosis within tumor is more commonly seen in pancreatic ductal adenocarcinoma or a neuroendocrine tumor, PPL must be included in the differential diagnosis.
Figures and Tables
Fig. 1
Primary pancreatic lymphoma in a 27-year-old man.
A. Contrast enhanced MDCT shows poorly enhancing mass (arrowheads) of 5 cm in diameter with low internal attenuation (curved arrow) in the pancreas head, with moderate dilatation of the pancreatic duct (arrow) and both intrahepatic bile ducts.
B. T1-weighted MR image shows a hypointense homogeneous mass (arrowheads) with an internal hyperintense area (curved arrow) and severe dilatation of the pancreatic duct (arrow).
C. T2-weighted MR image demonstrates a hyperintense mass with an internal hyperintense area (curved arrow) and severe dilatation of the pancreatic duct (arrow).
D. Gadolinium-enhanced T1-weighted coronal MR image shows a homogeneous enhancing mass (arrowheads) with a non-enhancing area (asterisk) demonstrating severe dilatation of the distal pancreatic duct (curved arrow) and common bile duct (arrow).
E. ERCP demonstrates moderate to severe dilatation of the upstream pancreatic duct (arrowhead), both intrahepatic bile ducts, and the common bile duct (arrow).
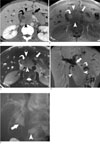
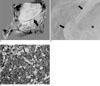
Fig. 2
Photographs of the tumor.
A. photograph of the resected specimen shows a well circumscribed whitish mass at the pancreatic head with invasion of the pancreatic duct (arrow) and duodenal wall (arrowhead).
B. Photomicrograph shows focal coagulative necrosis (asterisk) with a well circumscribed margin (arrows), which was considered as hemorrhage on a preoperative MRI (H & E, × 20).
C. The tumor cells have an immunoreactivity for CD20, which is suggestive of lymphoma (immunostaining, × 200).
References
1. Lin H, Li SD, Hu XG, Li ZS. Primary pancreatic lymphoma: Report of six cases. World J Gastroenterol. 2006; 12:5064–5067.
2. Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg. 1961; 49:80–89.
3. Van Beers B, Lalonde L, Soyer P, Grandin C, Trigaux JP, De Ronde T, et al. Dynamic CT in pancreatic lymphoma. J Comput Assist Tomogr. 1993; 17:94–97.
4. Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: differential aspects. AJR Am J Roentgenol. 2000; 174:671–675.
5. Prayer L, Schurawitzki H, Mallek R, Mostbeck G. CT in pancreatic involvement of non-hodgkin lymphoma. Acta Radiol. 1992; 33:123–127.
6. Shirkhoda A, Ros PR, Farah J, Staab EV. Lymphoma of the solid abdominal viscera. Radiol Clin North Am. 1990; 28:785–799.
7. Buetow PC, Miller DL, Parrino TV, Buck JL. Islet cell tumors of the pancreas: clinical, radiologic, and pathologic correlation in diagnosis and localization. Radiographics. 1997; 17:453–472.
8. Semelka RC, Ascher SM. MR imaging of the pancreas. Radiology. 1993; 188:593–602.
9. Saif MW. Primary pancreatic lymphomas. JOP. 2006; 7:262–273.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download