Abstract
Inflammatory myofibroblastic tumor is a benign lesion in pathology. However it may be associated with the invasion of adjacent structures mimicking malignancy. We describe a case of aggressive pulmonary inflammatory myofibroblastic tumor with chest wall invasion and spine destruction.
An inflammatory myofibroblastic tumor is a benign inflammatory tumefaction of unknown etiology. It is relatively rare but could occur at various locations throughout the body (1). Inflammatory myofibroblastic tumors are generally considered a benign mass and include inflammatory cells, histiocytes, and fibroblasts (1). Nevertheless, aggressive features that may mimic a malignant tumor have been described. We present a malignancy mimicking inflammatory myofibroblastic tumor in the left upper lobe which extended to the chest wall and spinal canal with bony destruction.
A 46-year-old man presented with pain on the left shoulder and upper back for three months. He had numbness on both lower legs and had difficulty walking and had no previous medical issues.
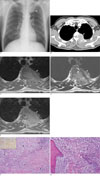
A chest radiograph showed an ill-defined increased opacity of about 6cm in diameter located in the left upper lung zone above the aortic arch (Fig. 1A). The contrast enhanced computed tomography (CT) scans showed strongly enhanced soft tissue mass in the apicoposterior segment of the left upper lobe, which extended to the chest wall and left 3rd, and 4th rib destruction (Fig. 1B). The mass also extended into the spinal canal with thecal sac compression. T1-weighted images of magnetic resonance (MR) images demonstrated a heterogeneous mass with a signal intensity slightly greater than that of skeletal muscle (Fig. 1C). It was slightly hyperintense on T2-weighted images (Fig. 1D), and moderately enhanced on gadolinium-enhanced images (Fig. 1E). The signal intensity of the bone marrow of T3 and T4 vertebral bodies was diffusely decreased on T1- and T2-weighted images.
Laboratory examinations revealed increased free kappa light chain (35.70 mg/L) and increased serum free lambda light chain (35.20 mg/L). The serum kappa to lambda ratio was within normal limits (normal reference range, 0.26-1.65). The serum protein electrophoresis showed increased gamma-globulin fraction with a polyclonal feature. Thus, we could rule out a plasmacytoma, a malignant plasma cell tumor.
A percutaneous needle biopsy was performed. Microscopic examination revealed a marked infiltration of plasma cells. Subsequently, a surgical biopsy with debulking operation was done. The mass could not be completely removed because of its invasion into the spinal canal and vertebral body. A histologic examination demonstrated that the lesion was composed of inflamed fibrous connective tissue showing compact fascicular spindle cell proliferation (Fig. 1F) and trabecular bone with massive plasmacytic infiltration (Fig. 1G). Immunophenotypically, the myofibroblasts were negative for ALK1 (anaplastic large cell lymphoma kinase 1) and positive for desmin and smooth muscle actin. The plasma cells were polyclonal for the immunoglobulin light chain.
Radiotherapy was administered (45 Gy), with no corticosteroid therapy and the patient had no significant side effects except for mild back pain.
An inflammatory myofibroblastic tumor is a relatively rare benign inflammatory process that can occur in various organs of the body, lung, orbit, nasal sinuses, liver, spleen, pancreas, bowel, kidney, urinary bladder, testis, heart, lymphatic system, skin and so on (1). However, the lung is the most frequently involved organ (2). The pathogenesis of the inflammatory myofibroblastic tumor is uncertain. Histopathologic examination reveals infiltration of polygonal lymphocytes, plasma cells, and macrophages without the evidence of atypia, and is composed of various inflammatory cells. Therefore, an inflammatory myofibroblastic tumor is thought to be an inflammatory process rather than a neoplasm (3).
The patients who have a pulmonary inflammatory myofibroblastic tumor frequently complain of nonspecific symptoms such as cough, fever, dyspnea, cyanosis, hemoptysis, and chest pain (34).
The vast majority of pulmonary inflammatory myofibroblastic tumors occur as solitary, well-defined masses. They typically occur in an intraparenchymal location, but may occur in the trachea and the bronchi (5). Nevertheless, extrapulmonary extensions suggestive of malignancy have been described in some previous reports (23) and have been found to invade the mediastinum and diaphragm (2), as well as the thoracic vertebra (3). The case we report here showed an ill-defined lung mass with infiltration to the adjacent structures including vertebrae and ribs with imaging findings indicating severe destruction. However, no laboratory evidence of plasmacytoma was found, and immunoperoxidase reactions for light chains evidently demonstrated the polygonal nature of the plasma cells.
On chest radiograph, pulmonary inflammatory myofibroblastic tumor typically appears as a solitary, peripheral, sharply circumscribed, lobulated mass (6). CT features including contrast enhancement and the patterns of calcification have been reported as variable and nonspecific (5). However, they most commonly appeared with heterogeneous attenuation and enhancement on CT scans. On T1-weighted images, these tumors have intermediate signal intensity, and have high signal intensity on T2-weighted images (6). In our case, the mass had an ill-defined border and showed homogeneous enhancement on CT scans. On MR image, the mass was hypointense on T1-weighted images, iso- to slight hyperintense on T2-weighted images and markedly enhanced after gadolinium was administered.
The radiologic differential diagnosis for an inflammatory myofibroblastic tumor occurring as a solitary pulmonary nodule includes primary or secondary neoplasm, hamartoma, chondroma, hemangioma, granuloma, and pulmonary sequestration (6). However, the differential diagnosis may be difficult in a case with aggressive features. In our case, the initial diagnosis was a malignant tumor, which originated from lung or chest wall.
Complete surgical resection is considered the most effective treatment of an inflammatory myofibroblastic tumor (7). Steroid therapy or radiation therapy could be effective in surgically unresectable cases (4). Our case was impossible to make the complete resection because of invasion to the adjacent structures. Our case study underwent radiation therapy after an excisional biopsy.
In conclusion, an inflammatory myofibroblastic tumor is a benign lesion of unknown etiology. However, it may demonstrate locally aggressive behavior mimicking a malignant tumor. We reported an extremely rare case of an inflammatory myofibroblastic tumor that invaded into the chest wall and spinal canal.
Figures and Tables
Fig. 1
Pulmonary inflammatory myofibroblastic tumor with bone destruction and spinal canal invasion in a 45-year-old man.
A. Chest radiograph shows ill-defined increased opacity (arrow) in the left upper lobe above the aortic arch.
B. Contrast-enhanced axial CT scan reveals a well-enhanced soft tissue mass (arrow) in the apicoposterior segment of the left upper lobe which extended to the chest wall with left 3 rd, and 4th rib destruction. The mass also invaded into the spinal canal with cord compression.
C-E. Axial MR image shows a heterogeneous mass (arrow) with signal intensity slightly greater than that of the skeletal muscle on a T1-weighted image, slightly hyperintense on a T2-weighted image and moderately enhanced on a gadolinium-enhanced T1-weighted image.
F. Microscopically, there is fascicular proliferation of plump spindle cells featuring myofibroblast (arrows) and inflammatory infiltrates mainly composed of plasma cells (arrow heads) (H & E, ×200). Inset shows myofibroblast with positive staining for smooth muscle actin.
G. The marrow space of bone show extensive infiltration of mature plasma cells (arrow heads) along with loose myofibroblastic (arrows) proliferation.
References
1. Boutarbouch M, Arkha Y, Rifi L, Derraz S, El Ouahabi A, El Khamlichi A. Intradural cervical inflammatory pseudotumor mimicking epidural hematoma in a pregnant woman: case report and review of the literature. Surg Neurol. 2008; 69:302–305.
2. Corneli G, Alifano M, Forti Parri S, Lacava N, Boaron M. Invasive inflammatory pseudo-tumor involving the lung and the mediastinum. Thorac Cardiovasc Surg. 2001; 49:124–126.
3. Hong HY, Castelli MJ, Walloch JL. Pulmonary plasma cell granuloma inflammatory pseudotumor with invasion of thoracic vertebra. Mt Sinai J Med. 1990; 57:117–121.
4. Kim JH, Cho JH, Park MS, Chung JH, Lee JG, Kim YS, et al. Pulmonary inflammatory pseudotumor-a report of 28 cases. Korean J Intern Med. 2002; 17:252–258.
5. Bahadori M, Liebow AA. Plasma cell granulomas of the lung. Cancer. 1973; 31:191–208.
6. Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003; 23:719–729.
7. Scott L, Blair G, Taylor G, Dimmick J, Fraser G. Inflammatory pseudotumors in children. J Pediatr Surg. 1988; 23:755–758.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download