Abstract
Purpose
The purpose of this study is to describe the characteristic features of cerebral sparganosis with live worm on CT and MRI as compared with the characteristic CT and MRI features of degenerated cerebral worms.
Materials and Methods
Twelve patients with surgically-proven live worms (the live group) and 13 patients with degenerated worms (the degenerated group) were included in this study. We retrospectively reviewed the CT scans of all the patients and the MR images of 19 patients.
Results
The live group showed highly attenuated lesion (50-70 HUs for the CT number) on precontrast CT with surrounding enhancement extending to the cortical surface, a mass effect and change of the location and shape of the enhancing lesion on the follow-up images, whereas the degenerated group showed punctate calcification (more than 130 HUs) with surrounding enhancement and cerebromalacia in 77% of the subjects (p<.01). The highly attenuated lesion had an amorphous nodular or nodulotubular shape, and these lesions were slightly hyperintense on the T1-weighted MR images and hypointense on the T2-weighted MR images.
Conclusion
In patients with cerebral sparganosis, live worm infection is highly suggested if there is a focal amorphous hyperattenuated lesion on the precontrast CT with a surrounding mass effect, irregular nodulotubular enhancement extending to the pial surface and change of the enhancing lesion on the follow-up images.
Human cerebral sparganosis is a rare parasitic disease that is caused by a migrating plerocercoid larva (sparganum) of the genus Spirometra (123). This disease has been reported worldwide, but it is most common in East Asia (23456789). The CT and MR imaging features of cerebral sparganosis have been well described (1011121314). However, no specific imaging features of cerebral sparganosis that indicate the presence of a live worm have been yet reported, despite its clinical importance, because of the rarity of surgically-confirmed cases with extraction of a live worm. The best way to treat symptomatic cerebral sparganosis is surgically removing both the worm and the granuloma (15). Especially, a live sparganum worm usually migrates in the brain parenchyma and causes progressive neuronal damage, so urgent removal of the live worm is crucial to prevent further neurologic deficits (81016). Thus, it is important to determine whether the worm is alive or not for the treatment planning.
During the last thirteen years, we encountered 12 patients with cerebral sparganosis with a live spargana worm and 13 patients with a degenerated worm at surgery. This report describes the characteristic CT and MR imaging findings of cerebral sparganosis in groups of patients with live and degenerated worms.
During a thirteen years period (1988-2000), we encountered a group of 12 patients (9 men and 3 women, aged 6-60 years, mean age: 32 years) with cerebral sparganosis with a live worm and a group of 13 patients (11 men and 2 women, aged 19-58 years, mean age: 38 years) with cerebral sparganosis and a degenerated worm. CT was done for all the patients and MR imaging was performed for 19 patients with postcontrast enhancement (7 in the live group and 12 in the degenerated group). Ten patients underwent follow-up CT or MR imaging at 2-39 month intervals (4 in the live group and 6 in the degenerated group). For all the patients, the preoperative diagnosis was made on the basis of the clinical history, the CT and MR imaging findings and a positive enzyme linked immunosorbent assay (ELISA) test for sparganunm-specific immunoglobulin G (IgG) antibody in both the serum and cerebrospinal fluid. A live sparganum was confirmed at surgery in all the patients of the live group, and inflammatory granuloma without a live worm was found at surgery in 9 patients of the degenerated group. Four patients in the degenerated group did not undergo surgery, but their lesions did not change except for observing cerebromalacia on the follow up CT or MR images.
We retrospectively analyzed the imaging features to determine if there were any differential imaging features between the live and degenerated groups with special attention being given to the following: the presence or absence of a highly attenuated lesion that ranged from 50-70 HUs in the center of the enhancing lesion for the CT number on the precontrast CT, the MR signal intensity of the lesion, the features of the enhancing lesion, the presence or absence of a mass effect (gyral effacement, ventricular compression), cerebromalacia or atrophy nearby or in an area remote from the enhancing lesion and the serial change of the enhanced lesion on follow-up studies. Statistical analysis was done using the Mantel-Haenszel Chi-square test.
The differential imaging features are summarized in Table 1. On the precontrast CT scan, a highly attenuated lesion was always seen in the center of the enhancing lesion in all the patients of the live worm group, while this was absent in the degenerated worm group (p<0.01). The highly attenuated lesion was located in the cortex or subcortical white matter, it appeared as an irregular nodular or it was tubular in shape (Fig. 1A) and it was approximately 0.5 cm to 3.0 cm in length. There was always enhancement surrounding the high attenuated lesion, gyral swelling and a localized area of low attenuation surrounding the highly attenuated lesion on the CT scan (Fig. 1B). The precontrast CT scan of the degenerated group frequently showed punctate calcifications (Fig. 2A). These punctate calcifications were located at the center of the enhancement in 77% (10/13) of the degenerated group. On the postcontrast images, enhancing lesion was seen in all the cases. The enhancing lesions had a variety of appearances: amorphous, nodular, serpentine tubular or ring-shaped (Fig. 1B). Interestingly, the enhancing lesions had continuity with a cortical surface in all the live cases, while only 25% of the degenerated cases showed this feature (p<0.01).
On MR imaging, the highly attenuated lesions were all hypointense (Fig. 1D) on the T2-weighted images (T2WI), and 57% (4/7) and 43% (3/7) of the high attenuated lesions were slightly hyperintense or isointense, respectively, relative to the brain cortex on the T1-weighted images (T1WI). All the enhancing lesions of the degenerated group showed isointensity on the T1WI (Fig. 2B) and hypointensity on the T2WI (Fig. 2C). On the follow-up studies, all the subjects in the live group showed changes of location and shape of the enhancing lesions (Fig. 1C, G) while the degenerated group showed no change of location (p<0.01). In one case, a previously enhancing lesion disappeared with remaining cortical atrophy and adjacent ventricular dilatation, while a new enhancing lesion appeared at a higher cortical area on the follow-up imaging (Fig. 1). Localized gyral effacement was always seen in all the subjects of the live group, while most of the subjects of the degenerated group showed regional atrophy with ventricular dilatation (Fig. 2) (p<0.01). However, an area of atrophy remote from the enhancing lesion was frequently seen in the live worm group of subjects (83%).
The characteristic CT findings of cerebral sparganosis have been well described by Chang et al. (10), which were white matter hypoattenuation with adjacent ventricular dilatation, irregular or nodular enhancing lesion and small punctate calcifications (101112). These findings might reflect both the live and degenerated stages of cerebral sparganosis. In the present study, all the patients of the live group showed a highly attenuated lesion on the precontrast CT. There has been no study that has mentioned this highly attenuated lesion, and this type of lesion seemed to be regarded in the previous reports as calcification or petechial hemorrhage (101112131415). However, we consider this lesion to be a live worm because of the following reasons. First, the CT numbers of the highly attenuated lesions ranged from 50 to 70 HU, which is lower than that of calcification or acute hemorrhage. In our unpublished experimental study with cats, the worm showed the same range of CT numbers. Second, in our series, some of the highly attenuated lesions totally disappeared or changed in location or shape, so it is unlikely to be calcification. Third, the shape and size of the highly attenuated lesion looked similar to those of the worm itself and on the surgical fields a live spargana worm was found at the site corresponding to the highly attenuated lesion, and neither hemorrhage nor calcification was found.
This lesion was slightly hyperintense or isointense relative to the brain cortex on the T1WI, and it was hypointense on the T2WI. The reason why the worm showed high attenuation on the precontrast CT and such MR signal intensities is not fully understood. The existence of high proteinaceous secretory granules, corpuscles or muscles in the worm might contribute to this CT attenuation and MR intensity (1011121314). Besides the highly attenuated lesion, punctate calcifications were also seen in both groups, and the attenuation of the punctate calcifications appeared much higher than that of the worm on CT. In half of the patients with live worm, punctate calcifications were seen in an area remote from the enhancing lesion along with cortical atrophy and ventricular dilatation, which showed neither surrounding enhancement nor a mass effect, which was in contrast to the highly attenuated lesion. This punctate calcification may have resulted from degenerated calcospherules or calcified muscle bundles in the fragmented worm (256789). However, on the CT of the degenerated group that showed punctate calcification (77%), there was enhancement surrounding the punctate calcification without gyral swelling. The highly attenuated lesion can be differentiated from punctate calcification by the CT HU number and the shape and presence of a surrounding mass effect. Thus, CT examination seems to be more useful than MR imaging for detecting a live worm.
On the postcontrast CT or MR images, there was always enhancing lesion irrespective of live or degenerated worm. The enhancing lesions in the patients of the live group always showed enhancement just around the highly attenuated lesion, whereas the degenerated group showed enhancing lesion just around the punctate calcification (77%) or enhancement without visible central highly attenuated foci (23%).
The enhancing lesions appeared as irregular nodular, tubular, beaded or conglomerated ring-shaped. The enhancing lesions in the patients of the live group always showed extension to the pial surface, while that of the degenerated group showed this feature in only 25% of the subjects. On the microscopic findings of our surgical specimens, granulomas were formed just around the fragmented body of the worm. The worm was surrounded by a dense collagenous wall and next to the collagenous wall there was a zone of granulation tissue in which many newly formed capillaries and infiltrated macrophages and lymphocytes were found (23). This highly vascular granulation tissue zone is presumably reflected by contrast enhancement (1213). The enhancing areas adjacent to the pial surface are considered to be the tract of previous penetration by the worm. We suggest that the invasion of the worm to the brain parenchyma starts from the pial surface. On MR imaging, extension of the enhancing lesion to the cortical surface was always seen on least on one of the multiplanar images. On the follow-up images of the live group, the irregular enhancing lesion usually changed in both location and shape and sometimes in the degree of enhancement, suggesting migration of a live worm. Cortical atrophy remote from the enhancing lesion was more frequently seen in the live group (83%) than that in the degenerated group (31%). The cortical atrophy and ipsilateral ventricular dilatation are suggested to be the chronic sequelae of previous migration of a live spargana worm.
From the imaging findings of the patients with cerebral sparganosis with a live worm, we can postulate the serial pathologic changes of cerebral sparganosis. First, the invasion route of the sparganum to the brain parenchyma may be direct penetration from the pial surface. Second, in an acute stage of cerebral sparganosis when the worm is alive, it produces local inflammation, resulting in a surrounding mass effect and irregular nodular enhancement (9). Third, after passage or migration of the live sparganum worm, the worm's previous cortical or white matter site becomes atrophied with ipsilateral ventricular dilatation (1011121314). Sometimes in these atrophied areas, punctate calcifications may remain with surrounding enhancement.
In conclusion, the presence of amorphous nodular or tubular shaped highly attenuated lesion on precontrast CT with surrounding irregular nodulotubular enhancement extending to the pial surface, localized gyral swelling and serial changes of the enhancing lesion's shape and location on the follow-up imaging strongly suggest that a patient with cerebral sparganosis has a living worm in their brain.
Figures and Tables
Fig. 1
A 29-year-old male patient with a live worm.
A. On the precontrast CT, focal nodular highly attenuated lesion (arrow) is seen in the right anterior frontal cortex with surrounding low density. Note the mildly compressed ipsilateral frontal horn of the lateral ventricle.
B. On the postcontrast CT, the ring-shaped densely enhancing lesion (arrow) is seen just around the highly attenuated lesion.
C. On the postcontrast CT six month later, the previously enhancing lesion totally disappeared and cortical atrophy and ipsilateral ventricular dilatation remained (arrow).
D. On the T2-weighted MR image (at the same time as for Fig. C), the irregular nodular-tubular low signal intensity lesion (black arrow) with surrounding high signal intensity is seen in the right high frontal cortex.
E. On the T1-weighted MR image, this lesion (arrow) shows iso-signal intensity.
F. On the postcontrast T1-weighted MR image, the irregular nodulotubular shaped enhancing lesion (arrow) is well visualized from the pial surface to the white matter in the right frontal lobe.
G. On the postcontrast T1-weighted coronal MR image 2 month later, the shape of the enhancing lesion (arrow) is slightly changed.
H. On the surgical field, a live spargana worm (arrow) was found at the site corresponding to the enhancing lesion on MR.
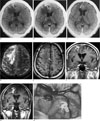
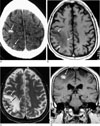
Fig. 2
A 52-year-old male patient with a degenerated worm.
A. On precontrast CT, irregular punctate calcifications (arrows) are seen in the high frontoparietal cortex and subcortical white matter with no surrounding low density.
B, C. On the follow-up MR 39 months later, localized cortical atrophy (white arrows) is seen with low signal intensity on the T1-weighted image (B) and high signal intensity on the T2-weighted image (C). Note the focal low signal intensity lesion in Fig C (black arrow), which seems to be the calcified lesion seen on CT.
D. After gadolinium injection, the ring shaped focal enhancing lesion (arrow) is seen with surrounding atrophy.
References
1. Muller JF. The biology of Spirometra. J Parasitol. 1974; 60:3–14.
2. Cho SY, Bae JH, Seo BS, Lee SH. Some aspects of human sparganosis in Korea. Korean J Parasitol. 1975; 13:60–77.
3. Chi JG, Chi HS, Lee SH. Histopathologic study on human sparganosis. Korean J Parasitol. 1980; 18:15–23.
4. Anegawa S, Hayashi T, Ozuru K, kuramoto S, Nishimura K, Shimizu T, et al. Sparganosis of the brain: case report. J Neurosurg. 1989; 71:287–289.
5. Anders K, Foley K, Stern E, Brown WJ. Intracranial sparganosis: an uncommon infection - case report. J Neurosurg. 1984; 60:1282–1286.
6. Yamashita K, Akimura T, Kawano K, Wakuta Y, Aoki H, Gondou T. Cerebral sparganosis mansoni: report of two cases. Surg Neurol. 1990; 33:28–34.
7. Mitchell A, Scheithauer BW, Kelly PJ, Forbes GS, Rosenblatt JE. Cerebral sparganosis: case report. J Neurosurg. 1990; 73:147–150.
8. Holodniy M, Almenoff J, Loutit J, Steinberg GK. Cerebral sparganosis: case report and review. Rev Infect Dis. 1991; 13:155–159.
9. Okamura T, Yamamoto M, Ohta K, Matsuoka T, Uozumi T. Cerebral sparganosis mansoni-case report. Neurol Med Chir (Tokyo). 1995; 35:909–913.
10. Chang KH, Chi JG, Cho SY, Han MH, Han DH, Han MC. Cerebral sparganosis: analysis of 34 cases with emphasis on CT features. Neuroradiology. 1992; 34:1–8.
11. Chang KH, Cho SY, Chi JG, Kim WS, Han MC, Kim CW, et al. Cerebral sparganosis: CT characteristics. Radiology. 1987; 165:505–510.
12. Chang KH, Cho SY, Hesslink JR, Han MH, Han MC. Parasitic diseases of the central nervous system. Neuroimaging Clin of North Am. 1991; 1:159–178.
13. Moon WK, Chang KH, Cho SY, Han MH, Cha SH, Chi JG, et al. Cerebral sparganosis: MR imaging versus CT features. Radiology. 1993; 188:751–757.
14. Kradel J, Drolshagen LF, MacDade A. MR and CT findings in cerebral sparganosis. J Comput Assist Tomogr. 1993; 17:989–990.
15. Kim DG, Paek SH, Chang KH, Wang KC, Jung HW, Kim HJ, et al. Cerebral sparganosis: clinical manifestations, treatment, and outcome. J Neurosurg. 1996; 85:1066–1071.
16. Song T, Wang WS, Zhou BR, Mai WW, Li ZZ, Guo HC, et al. CT and MR characteristics of cerebral sparganosis. AJNR Am J Neuroradiol. 2007; 28:1700–1705.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download