Abstract
Inflammatory myofibroblastic tumor is a rare benign condition of an unknown etiology and it may be clinically misdiagnosed as a malignant tumor. We examined a 28-year-old male with a slowly-growing mass in his left thigh. The MR images showed a relatively well-defined, intermuscular mass lesion with heterogeneous enhancement. The histopathologic examination of the resected specimen revealed inflammatory myofibroblastic tumor. We report here on the MR findings of inflammatory myofibroblastic tumor of the thigh in this patient.
Inflammatory myofibroblastic tumor is a rare histologically benign mass that is characterized by a variably cellular, spindle cell proliferation in a myxoid to collagenous stroma with a prominent inflammatory infiltrate that is primarily composed of plasma cells and lymphocytes, with occasionally admixed eosinophils and neutrophils (1). Inflammatory myofibroblastic tumor most commonly involves the lung and the orbit, but it has been reported to occur in nearly every part of the body, from the central nervous system to the gastrointestinal tract (2).
To the best of our knowledge, the MR imaging of inflammatory myofibroblastic tumor located in the intermuscular portion of the thigh has not been previously reported in the English medical literature. Therefore, we present an extremely rare case of inflammatory myofibroblastic tumor that originated in the thigh.
A 28-year-old male was referred to our hospital with a two-year history of a protruding mass in the medial aspect of the left thigh. The size of the mass had also recently increased. On physical examination, the mass was noted to be slightly movable. The mass measured approximately 7 × 7 cm. There was no tenderness or symptoms of systemic disease. The patient denied ever having suffered any trauma to the thigh. The overlying skin and subcutaneous tissues were normal.
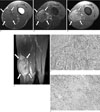
On plain radiography, the mass revealed no calcification (not shown). MR imaging was performed using a 1.5-T MR system (Symphony, Siemens Medical Systems, Erlangen, Germany). The mass lesion showed heterogeneously low signal intensity on the fast spin echo (FSE) T1-weighted image (Fig. 1A). On the FSE fatsaturated T2-weighted image, the mass showed heterogeneously low signal intensity with multifocal dark signal intensities (Fig. 1B). These dark signal intensities were regarded as hemorrhage. There was also prominent enhancement with multiple non-enhanced spots in the mass following intravenous injection of MR contrast material (Figs. 1C, D). The radiologic impression was hemangiopericytoma, malignant fibrous histiocytoma (MFH), malignant peripheral nerve sheath tumor (MPNST), fibrosarcoma and deep fibromatosis.
Excisional biopsy was performed, and the mass was then completely removed from the thigh. There was no evidence of invasion to the muscles of the left thigh. There were no tumor cells seen in the resected margins on the frozen section.
The gross specimen consisted of an ovoid, firm mass. The cut surface of the mass was whitish gray and trabeculated with hemorrhagic foci. Microscopic examination of the hematoxylin and eosin (H & E) stained tissue specimen (Fig. 1E) showed that mixed inflammatory cells, such as lymphocytes and plasma cells, were infiltrated between the spindle cells. On immunohistochemical staining, the specimen showed a positive reaction for smooth muscle actin (SMA), vimentin and anaplastic lymphoma kinase (ALK) (Fig. 1F), and a negative reaction for cytokeratin (CK), S100-protein and desmin. Therefore, the overall findings were consistent with inflammatory myofibroblastic tumor. The patient has had no signs of lesion recurrence and he has remained healthy for the six months since the operation.
The term "inflammatory pseudotumor" has been used to describe a wide range of reactive and neoplastic lesions, including inflammatory myofibroblastic tumor, pseudosarcomatous myofibroblastic proliferations of the genitourinary tract, infectious and reparative processes, and inflammatory pseudotumors of lymph node, spleen and orbit (1). Of these entities, inflammatory myofibroblastic tumor has recently emerged as a distinct entity that shows characteristic clinical, pathological and molecular structures. At presentation, it is frequently misinterpreted as a malignancy.
The pathogenesis of inflammatory myofibroblastic tumor is unclear, that is, whether it is inflammatory reactive process or a neoplastic process. It is considered by some investigators to be an aberrant reactive or inflammatory response to local cytokines (3). On the other hand, rearrangements involving the anaplastic lymphoma kinase (ALK) locus on chromosome 2p23 have been documented in both pulmonary and extrapulmonary inflammatory myofibroblastic tumors, and this provides further support for the neoplastic nature of these lesions (4).
Histologically, inflammatory myofibroblastic tumor is composed of myofibroblastic spindle cells that are mixed with a prominent infiltration of lymphocytes, plasma cells and acute inflammatory cells (5). On immunohistochemistry, approximately 50% of inflammatory fibroblastic tumors are positive for ALK (4). Immunohistochemical staining for ALK is helpful if this is present, but its absence does not exclude the diagnosis of inflammatory myofibroblastic tumor and particularly in adults (1).
The MR imaging findings of inflammatory myofibroblastic tumors that have occurred at various sites have been reported (678). However, the MR imaging findings of an inflammatory myofibroblastic tumor in the intermuscular portion of the thigh have not been previously reported. Our case shows that inflammatory myofibroblastic tumor can appear on MR imaging as a solid mass with hemorrhage. The signal intensity showed heterogeneously low signal intensity on the T1- and T2-weighted images. Han et al. (6) suggested that T2 hypointensity of a soft-tissue lesion, which may be explained by its relative lack of both free water and mobile protons within fibrotic lesions, is characteristic of fibrosing inflammatory pseudotumor. On our T1-weighted images obtained after injecting MR contrast material, the intermuscular thigh mass showed marked enhancement except in the areas of hemorrhage. However, this enhancement pattern on MR imaging may have a variable appearance according to the relative amount of fibrous tissue and cellular material in the mass (7).
The differential diagnosis in our case was hemangiopericytoma, MFH, MPNST, fibrosarcoma and deep fibromatosis. For the case of hemangiopericytoma, it is seen as a large mass with prominent serpentine vessels on MR imaging. MFH, MPNST, fibrosarcoma and deep fibromatosis show heterogeneous signal intensity on MR imaging depending on the variable amounts of collagen, myxoid tissue, necrosis and hemorrhage. Heterogeneously low signal intensity on T2-weighted imaging may be seen in MFH, MPNST, fibrosarcoma and deep fibromatosis (9), yet these findings are similar to those of our case. Therefore, it is impossible to distinguish these other diseases from inflammatory myofibroblastic tumor solely on the basis of the imaging findings.
The biologic potential of inflammatory myofibroblastic tumor is highly variable, although it generally has an innocuous course. Because of the similarity of MR imaging between inflammatory myofibroblastic tumor and other malignant soft tissue tumors, most of the reported inflammatory myofibroblastic tumors were treated surgically, and surgical removal was curative in most cases (78).
In conclusion, inflammatory myofibroblastic tumor is a rare condition and it may be included in the differential diagnosis of an intermuscular thigh mass that shows heterogeneous signal intensity and enhancement on MR imaging.
Figures and Tables
Fig. 1
A 28-year-old male with a 5×5×8 cm mass in the left thigh.
A. The axial FSE T1-weighted MR image (TR/TE = 606/20) shows heterogeneously low signal intensity in the mass, and the mass was located in the intermuscular space between the vastus medialis, sartorius, gracilis and adductor muscles.
B. The axial FSE fat-saturated T2-weighted MR image (TR/TE = 3510/107) shows heterogeneously low signal intensity with multifocal dark signal intensities in the mass.
C, D. The axial and coronal fat-saturated FSE T1-weighted MR images (TR/TE = 967/20) obtained after MR contrast injection show diffusely heterogeneous enhancement with multifocal non-enhanced portions.
E. H & E staining shows that mixed inflammatory cells, such as lymphocytes and plasma cells, were infiltrated between the spindle cells (× 200).
F. Immunohistochemical staining for anaplastic lymphoma kinase (ALK) shows a positive reaction (× 200).
Acknowledgments
We thank Bonnie Hami, Department of Radiology, University Hospitals of Cleveland, for her editorial assistance in the preparation of this manuscript.
References
1. Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008; 61:428–437.
2. Narla LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003; 719–729.
3. Pettinato G, Manivel JC, De Rosa N, Dehner LP. Inflammatory myofibroblastic tumor (plasma cell granuloma). Clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations. Am J Clin Pathol. 1990; 94:538–546.
4. Coffin CM, Patel A, Perkins S, Elenitoba-Johnson K, Perlman E, Griffin CA. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001; 14:569–576.
5. Bandwein MS, Som PM. Tumors and tumor-like conditions. In : Som PM, Curtin HD, editors. Head and neck imaging. 4th ed. St. Louis, MO: Mosby;2003. p. 250–251. p. 320–321. .
6. Han MH, Chi JG, Kim MS, Chang KH, Kim KH, Yeon KM, et al. Fibrosing inflammatory pseudotumors involving the skull base: MR and CT manifestations with histopathologic comparison. AJNR Am J Neuroradiol. 1996; 17:515–521.
7. Irie H, Honda H, Kaneko K, Kuroiwa T, Fukuya T, Yoshimitsu K, et al. Inflammatory pseudotumors of the spleen: CT and MRI findings. J Comput Assist Tomogr. 1996; 20:244–248.
8. Georgia JD, Lawrence DP, DeNobile JW. Inflammatory pseudotumor in the retrorectal space: CT and MR appearance. J Comput Assist Tomogr. 1996; 20:410–412.
9. Kransdorf MJ, Murphey MD. Vascular and lymphatic tumors / Benign fibrous and fibrohistiocytic tumors / Malignant fibrous and fibrohistiocytic tumors / Neurogenic tumors. In: Imaging of soft tissue tumors. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins;2006. p. 179–180. p. 217–231. p. 271–293. p. 337–348.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download