Abstract
Purpose
To identify radio-clinico-pathologic factors that result in false negative FDG uptake on 18F-FDG -PET/CT in the diagnosis of breast cancer.
Materials and Methods
We retrospectively reviewed a total of 140 breast lesions in 140 patients (mean age: 51.3 years) who underwent PET/CT for the staging of breast cancer from May 2007 to January 2008. All patients were divided as false negative (group 1, n=20) or true positive (group 2, n=120). A retrospective analysis was performed to analyze the statistical differences in clinico-pathologic factors between groups 1 and 2 using the Mann-Whitney U test, as well as the stepwise logistic regression analysis and the Chi-squared or Fisher's exact test.
Results
Estrogen receptor positivity, mass on mammography, carcinoma in situ, and size were significantly different between groups 1 and 2 (p < 0.05). A stepwise logistic regression analysis showed that the estrogen receptor positivity (odds ratio, 5.623; 95% confidence interval: 1.100, 28.746; p = 0.021) and carcinoma in situ (odds ratio, 6.900; 95% confidence interval: 1.151, 41.361; p = 0.026) were significant clinico-pathology variables associated with false negative PET/CT findings.
The combination of positron emission tomography (PET) and computed tomography (CT) are increasingly used for oncologic imaging. In particular, positron emission tomography with fluorine-18 fluorodeoxyglucose (18F-FDG PET/CT) is suggested as a more useful modality for accurate, non-invasive imaging in predicting the prognosis and staging of breast cancer (1234). The combination of positron emission tomography (PET) and computed tomography (CT) provides functional metabolic information (PET) and morphologic information. 18F-FDG PET/CT has been evaluated for primary breast cancer detection and diagnosis, staging of locoregional and distant sites, and monitoring the response to therapy in previous studies (567). Although 18F-FDG PET/CT is widely recognized as a useful diagnostic tool, it produces false-negative results in 12% of cancer cases (8).
The aim of this study was to identify radio-clinicopathologic factors that predict false negative FDG uptake results in breast cancer on 18F-FDG PET/CT.
We retrospectively reviewed a total of 140 breast cancers in 140 patients (mean age, 51.3 years: range, 28-86 years) from May 2007 to January 2008. All patients were histologically or cytologically confirmed as having breast cancer before undergoing 18F-FDG PET/CT. All patients were examined with 18F-FDG PET/CT for staging of breast cancer. This retrospective study was approved by the ethics review committee and informed consent was obtained from all patients. According to the results of 18F-FDG PET/CT, the patients were divided into two groups: Group 1 consisted of 20 patients who had negative results for the primary mass. Group 2 consisted of 120 patients who had positive results for the primary mass. The radio-clinico-pathologic factors including patient's age, tumor size, estrogen receptor (ER), progesterone receptor (PR), C-erb-B2, types of pathology and mammography findings, the inclusion of the mass (present, not present), clustered calcification (present, not present), and breast parenchyma composition (fatty breast, scattered fibroglandular tissue, heterogeneous fibroglandular tissue, dense breast) of group 1 and group 2 were retrospectively reviewed.
A bilateral mammography (MAMMOMAT NovationDR, Siemens Medical Solutions, Forchheim, Germany), including routine craniocaudal and mediolateral oblique views of the breasts, was performed. Findings were recorded prospectively according to BI-RADS by two radiologists who had 2 and 10 years of experience in performing mammographies. A mammography was performed at least 4 weeks before the other studies.
18F-FDG PET/CT was performed with a dedicated PET/CT scanner (Gemini, Philips Medical System, Milpitas, CA, USA), consisting of a germanium oxyorthosilicate full-ring PET scanner and a dual slice helical CT scanner. Standard patient preparation included at least 8 hours of fasting to attain a serum glucose level of less than 120 mg/dL before 18F-FDG administration. PET/CT imaging was performed 60 minutes after the injection of 4.5 MBq/Kg of 18F-FDG. At 60 minutes after administering 18F-FDG, low-dose CT (30 mAs, 120kV) covering an area from the base of the skull to the proximal thighs was performed for the purpose of attenuation correction and precise anatomical localization. Therefore, an emission scan was conducted in 3-dimensional mode. The emission scan time per bed position was 3 minutes; a total of 9 bed positions were acquired. PET data were obtained using a high resolution whole body scanner with an axial field of view of 18 cm. The average total PET/CT examination time was 30 minutes. After scatter and decay correction, PET data were reconstructed iteratively with attenuation correction and reoriented in axial, saggital, and coronal slices. The row action maximum-likelihood algorithm was used for 3-dimensional reconstruction.
For positive findings on the 18F-FDG PET/CT image, we relied on a visual focus of the PET image (a well-defined focus with uptake clearly greater than the surrounding background) and excluded the underlying morphologic CT information. A cut-off maximum standardized uptake value of 2.5 was applied to discriminate the positive and negative PET results. All 18F-FDG PET/CT images were directly reviewed on a computer workstation.
Formalin-fixed, paraffin-embedded sections of the resected mass were stained with hematoxylin-eosin (HE) and analyzed. Immunohistochemical analyses for the estrogen receptor (ER), progesterone receptor (PR), and c-erb-B2 (proto-oncogene) were performed using specific monoclonal antibodies.
Univariate and multivariate analyses were used for comparison of the two groups.
For the univariate analysis, age and size were compared using the Mann-Whitney U test; histologic results, mammography findings, ER, PR, and C-erb-B2 were compared by the Chi-squared test. P-values less than 0.05 were considered statistically significant.
For the statistical analysis, histologic results were divided into ductal carcinoma in situ and lobular carcinoma in situ versus invasive ductal carcinoma, invasive papillary carcinoma and mucinous carcinoma. Also, the breast composition among the mammography findings was divided into fatty breast and scattered fibroglandular tissue versus heterogeneous fibroglandular tissue and dense breast.
For the multivariate analysis, all factors were compared by stepwise logistic regression analysis.
The mean size of the masses were 1.8 cm (range, 1.2-3.5 cm) in group 1 and 2.4 cm (range, 1.5-5.4 cm) in group 2. The mean maximum standardized uptake values were 0.5 (range, 0-1.7) in group 1 and 6.1 (range, 2.6-9.6) in group 2. The other results are summarized in Table 1. Among all parameters, estrogen receptor positivity (p = 0.019), progesterone receptor positivity (p = 0.01), carcinoma in situ (p = 0.037), and the size of the mass (p = 0.012) were analyzed by Mann-Whitney test or Chi-square test and found to show a statistical difference between groups 1 and 2. A stepwise logistic regression analysis showed that estrogen receptor positivity (odds ratio, 5.623; 95% confidence interval: 1.100, 28.746; p = 0.021) and carcinoma in situ (odds ratio, 6.900; 95% confidence interval: 1.151, 41.361; p = 0.026) were significant clinico-pathology variables associated with a negative finding for 18F-FDG PET/CT in the diagnosis of primary breast cancer (Table 2).
Currently, a whole body CT, bone scintigraphy, and breast magnetic resonance imaging are used for the initial staging of the tumor and the detection of distant metastasis. 18F-FDG PET/CT is proposed as a single method that can replace these methods (9). 18F-FDG PET/CT provides functional, metabolic information, and morphologic information. However, Samson et al. reported that false negative results occurred in 12% of breast cancers (8).
Our results showed that the estrogen receptor positivity and carcinoma in situ were significant clinico-pathology variables associated with the negative findings of 18F-FDG PET/CT in the diagnosis of primary breast cancer. Therefore, in cases of estrogen receptor positivity and carcinoma in situ tumors, there is a high possibility that the primary mass and metastatic mass in the contralateral breast are not depicted on 18F-FDG PET/CT for stage workup.
Kumar et al. (10) reviewed 85 breast cancers and demonstrated that both tumor sizes of less than 10 mm and low tumor grade were significant predictors of a false-negative 18F-FDG PET/CT result. However, to our knowledge, there has been no report that estrogen receptor positivity is associated with a negative 18F-FDG PET/CT finding in the diagnosis of breast cancer.
Generally, estrogen receptor positive tumors are known to be less aggressive. Therefore, we considered that our result was attributed to the fact that in less aggressive tumors, glucose metabolism more slowly accelerates to meet the energy demand for tumor growth. Despite the fact that the estrogen receptor is still not completely understood, we hope that the knowledge gained from this study contributed to explaining 18F-FDG uptake rates.
Despite its several advantages, it is still questionable whether 18F-FDG PET/CT is useful for the stage workup of a tumor showing no 18F-FDG uptake. In fact, the current National Comprehensive Cancer Network practice guidelines recommend that routine chest imaging (chest radiography) should only be performed on patients with clinical stage I breast cancer. In patients with node-positive stage II and stage III disease, imaging typically consists of bone scanning and contrast-enhanced chest or abdominal CT. 18F-FDG PET/CT is recommended as an option for patients with either recurrent or stage IV disease, which in this setting, has been shown to be both sensitive and specific for metastases (111213).
There is widespread agreement that whole-body 18F-FDG PET/CT does not have a clinical role in detecting primary breast cancer, nor is it an alternative to histologic sampling to establish or exclude primary breast cancer because of the well-documented inability of 18F-FDG PET/CT to consistently demonstrate small and low-grade lesions (14). Therefore, other imaging modalities are required for the precise detection of breast cancer and metastasis evaluation. In some studies, the sensitivity and specificity of MR imaging were higher for BRCA mutation carriers (1516). In another study, a relatively high number of cancers (13 of 33 [39%]) were only visible on MR imaging, other than US and mammography (17).
Our study had three limitations: First, although the reference standard in this study was based on results from the combined analysis of detailed standardized pathologic reports and imaging studies, inaccuracies might have been introduced because of the retrospective nature of this study. Second, we did not include ultrasonography findings in the radio-clinico-pathologic factors, which are important diagnostic factors. Third, we considered the mass on the CT scan, without 18F-FDG uptake, as a negative finding. However, our study focused on 18F-FDG uptake, not morphologic information.
In conclusion, carcinoma in situ and ER positivity were significantly correlated with false negative FDG uptake in breast cancer on 18F-FDG PET/CT.
Figures and Tables
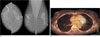 | Fig. 1Breast images of a 54-year-old woman with a 12 mm invasive ductal carcinoma in the right breast were not seen on 18F-FDG-PET/CT. The estrogen receptor was found to be positive.A. Bilateral craniocaudal (right) and mediolateral oblique (left) mammograms showed a round mass with a spiculated margin in the right upper outer quadrant (arrow).
B. 18F-FDG PET/CT showed no abnormally elevated FDG uptake rate for both breasts.
|
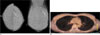 | Fig. 2Breast images of a 34-year-old woman with 9 mm carcinoma in situ in the left breast was not seen on 18F-FDG PET/CT. The estrogen receptor was negative.A. Bilateral craniocaudal (right) and mediolateral oblique (left) mammograms show clustered pleomorphic microcalcifications in upper outer quadrant of the left breast (arrow).
B. 18F-FDG PET/CT showed no abnormally elevated FDG uptake rate in both breasts.
|
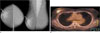 | Fig. 3Breast images of a 45-year-old woman with 42 mm invasive ductal carcinoma in the right breast was seen on 18F-FDG PET/CT. The estrogen receptor was found to be negative.A. Bilateral craniocaudal (right) and mediolateral oblique (left) mammograms showed an irregular-shaped mass with a partially indistinct margin in the subareolar region of the right breast (arrow).
B. 18F-FDG PET/CT showed an abnormal increase in FDG uptake in the upper region of the right breast. The maximum standardized uptake value was 5.2.
|
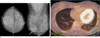 | Fig. 4Breast images of a 42-year-old woman with 35 mm invasive ductal carcinoma of the right breast was not seen on 18F-FDG PET/CT. The estrogen receptor was found to be positive.A. Bilateral craniocaudal (right) and mediolateral oblique (left) mammograms showed an oval-shaped mass with a partially indistinct margin in the subareolar region of the right breast (arrow).
B. 18F-FDG PET/CT showed no abnormally elevated FDG uptake rate in both breasts.
|
Table 1
Radio-Clinico-Pathologic Comparison of Two Groups by Univariate Analysis
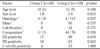
*Results were divided into ductal carcinoma in situ and lobular carcinoma in situ versus invasive ductal carcinoma, invasive papillary carcinoma and mucinous carcinoma.
†These findings are based on mammography.
‡Results were divided into fatty breast and scattered fibroglandular tissue versus heterogeneous fibroglandular tissue and dense breast on mammography.
Group 1: 20 patients who showed a negative result for the primary mass. Group 2: 120 patients who showed a positive result for the primary mass.
References
1. Tse NY, Hoh CK, Hawkins RA, Zinner MJ, Dahlbom M, Choi Y, et al. The application of positron emission tomographic imaging with fluorodeoxyglucose to the evaluation of breast disease. Ann Surg. 1992; 216:27–34.
2. Bruce DM, Evans NT, Heys SD, Needham G, BenYounes H, Mikecz P, et al. Positron emission tomography: 2-deoxy-2-(18F)-fluoro-D-glucose uptake in locally advanced breast cancers. Eur J Surg Oncol. 1995; 21:280–283.
3. Crippa F, Agrest R, Seregni E, Greco M, Pascali C, Bogni A, et al. Prospective evaluation of (18F) FDG positron emission tomography in the presurgical staging of the axilla in breast cancer: comparison between PET and postoperative pathology. J Nucl Med. 1998; 39:4–8.
4. Schirrmeister H, Kuhn T, Guhlmann A, Santjohanser C, Hörster T, Nüssle K, et al. Fluorine-18 2-deoxy-2-fluoro-D-glucose PET in the preoperative staging of breast cancer: comparison with the standard staging procedure. Eur J Nucl Med. 2001; 28:351–358.
5. Weir L, Worsley D, Bernstein V. The value of FDG positron emission tomography in the management of patients with breast cancer. Breast J. 2005; 11:204–209.
6. Eubank WB, Mankoff DA. Evolving role of positron emission tomography in breast cancer imaging. Semin Nucl Med. 2005; 35:84–99.
7. Lind P, Igerc I, Beyer T, Reinprecht P, Hausegger K. Advantages and limitations of FDG PET in the follow-up of breast cancer. Eur J Nucl Med Mol Imaging. 2004; 31:suppl 1. S125–S134.
8. Samson DJ, Flamm CR, Pisano ED, Aronson N. Should FDG PET be used to decide whether a patient with an abnormal mammogram or breast finding at physical examination should undergo biopsy? Acad Radiol. 2002; 9:773–783.
9. Sloka S, Hollett D, Mathews M. Cost-effectiveness of positron emission tomography in breast cancer. Mol Imaging Biol. 2005; 7:351–360.
10. Eubank WB, Mankoff D, Bhattacharya M, Gralow J, Linden H, Ellis G, et al. Impact of FDG PET on defining the extent of disease and on the treatment of patients with recurrent or metastatic breast cancer. AJR Am J Roentgenol. 2004; 183:479–486.
10. Kumar R, Chauhan A, Zhuang H, Chandra P, Schnall M, Alavi A. Clinicopathologic factors associated with false negative FDG-PET in primary breast cancer. Breast Cancer Res Treat. 2006; 98:267–274.
11. Aboagye EO, Price PM. Use of positron emission tomography in anticancer drug development. Invest New Drugs. 2003; 21:169–181.
12. Kamel EM, Wyss MT, Fehr MK, von Schulthess GK, Goerres GW. [18F]-fluorodeoxyglucose positron emission tomography in patients with suspected recurrence of breast cancer. J Cancer Res Clin Oncol. 2003; 129:147–153.
13. Rosen EL, Eubank WB, Mankoff DA. FDG PET, PET/CT, and Breast Cancer Imaging. Radiographics. 2007; 27:S215–S229.
15. Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005; 23:8469–8476.
16. Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005; 365:1769–1778.
17. Causer PA, Jong RA, Warner E, Hill K, Wong JW, Curpen BN, et al. Breast cancers detected with imaging screening in the BRCA population: emphasis on MR imaging with histopathologic correlation. Radiographics. 2007; 27:S165–S182.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download