Abstract
An 80-year-old man, who presented with a perianal mass, showed a multilocular mass with peripheral calcification in the retroanal region at CT. The MR imaging detected a mass invading into the posterior aspect of the external anal sphincter, and was shown as having a high T1 and T2 signal intensity with a different T1 signal intensity in each locule. After contrast injection, septal and peripheral enhancement of the tumor was observed. Surgery was performed and revealed a perianal mucinous adenocarcinoma. To the best of our knowledge, this is the first report describing the MR features of a perianal mucinous adenocarcinoma in the Korean literature. We described a case of perianal mucinous adenocarcinoma with an emphasis on the MR imaging features.
A perianal mucinous adenocarcinoma is a very rare tumor that may originate from an anal gland or a chronic perianal fistula. The tumor may spread around the anal canal and extend into the perianal soft tissue (1). Although there have been several studies describing perianal mucinous adenocarcinomas, few reports describe the MR imaging features. We presented a case of an 80-year-old man with a large perianal mucinous adenocarcinoma with an emphasis on the MR imaging findings. To the best of our knowledge, this is the first report describing the MR imaging features of a perianal mucinous adenocarcinoma in the Korean literature.
An 80-year-old man presented with a slowly-growing painful perianal mass that had been present for three years. The patient had a history of incision and drainage for an anal fistula about 10 years prior. A physical examination demonstrated the presence of an ulcerated and discharging lesion measuring 10 × 10 cm on the right posterolateral region of the anus. A proctocolonoscopy showed no abnormalities except for a hemorrhoid. Contrast-enhanced abdominopelvic CT images showed that the multilocular mass with multiple marginal calcifications in the retroanal region, was attached to the posterior aspect of the anal sphincter and extended to the skin of the buttock (Fig. 1A). A pelvic MR was performed to obtain additional information: T1-weighted MR images revealed that the multilocular mass had a slightly high signal intensity comparing to muscle, as well as a different internal signal intensity in each locule. The curvilinear high signal intensities, which were consistent with the marginal calcification on CT images, were also observed on the T1-weighted images along the margin of some of these locules (Fig. 1B). The T2-weighted images revealed a multilobular mass shown as fluid-like marked high signal intensity and a definite invasion of the tumor to the posterior aspect of the external anal sphincter. However, there was no observed invasion into the intrinsic anal sphincter or gluteal muscle, and no irregular thickening of the innermost layer of the anus representing the mucosa and submucosa or the epidermis and dermis (Figs. 1C, D). After the intravenous administration of the gadolinium contrast agent, the septal and peripheral enhancement was seen in the tumor (Figs. 1E, F). No enlargement of the pelvic and inguinal lymph nodes was noted, and a histological examination of a biopsy specimen taken from the ulcerated portion of the mass revealed the presence of a well-differentiated mucinous adenocarcinoma. A preoperative chest radiograph was found to be negative. A wide excision and sphincteroplasty were performed. The perianal defect was repaired using a fasciocutaneous flap. Grossly, the tumor was a huge cystic mass attached to the skin, measuring 10.5 × 11.0 × 5.0 cm. The cut surface on the section showed multiple cystic chambers containing gray yellowish to tan mucoid material. Microscopically, the glandular epithelial cells had hyperchromatic and atypical nuclei with nuclear stratification, which were consistent with a mucinous adenocarcinoma (Figs. 1G, H). In addition, an external sphincter invasion was identified; however, the resection margin of specimen was negative for the presence of a tumor. No adjuvant chemotherapy or radiation therapy was given since the patient refused further treatment. The patient was discharged in good condition at 24 days after surgery, and then he was lost to follow-up.
A perianal mucinous adenocarcinoma is an extremely rare malignancy that represents approximately 2% to 19% of all anal carcinomas, considering anal carcinomas represent 2% of all tumors of the gastrointestinal tract. The tumor may arise directly from an anal gland, a chronic fistulous tract, or from a duplicated duct; although the exact histogenesis of anal duct carcinomas is debatable. The mucin-producing anal gland extends from the crypt of Morgagni into the submucosa, proceeding at a tortuous course into the perianal soft tissue or even into the ischiorectal fossa. Dysplasia of the anal gland epithelium may lead to a perianal mucinous adenocarcinoma (1). In addition, a chronic perianal fistula lined by columnar epithelium may also progress into a mucinous adenocarcinoma. However, the perianal glands could be implicated in the formation of perianal fistulas and for an anal gland malignancy; and, it remains controversial whether a perianal anal carcinoma causes fistular formation or whether a chronic perianal fistula leads to an adenocarcinoma. This tumor is usually large at the time of presentation and has obliterated the normal anatomy, making it impossible to know the precise origin of the tumor. Despite a history of an anal fistula in our patient, it could not be determined whether the tumor had arisen from an anal fistula.
Demographically, a perianal mucinous adenocarcinoma is slightly more common in males than females, and cases occur at an average age of 55 years. Most tumors are located posteriorly to the anus. About half of patients present with a fistula. Common symptoms include a perianal lump, bleeding, pain, pruritus ani, and a change in bowel habit. The tumor could be initially neglected because of its tendency as a benign anal condition and coexisting perianal pathology such as an anal fistula. Therefore, although a mucinous adenocarcinoma is a low grade-malignant tumor, it is usually diagnosed at an advanced stage and the overall prognosis is poor (2). A high index of clinical suspicion is needed to make the diagnosis of perianal tumors, especially during the assessment of patients presenting with a perianal inflammatory condition.
During the process of diagnosis, a clinician can obtain a large amount of information for the characterization as well as the local extent of the perianal tumor or the presence of its distant metastasis from the use of imaging studies. In some previous reports concerning CT images, the perianal mucinous adenocarcinoma was described as an internally-calcified mass involving the posterior side of the rectum, or a multiple conglomerated cystic mass that surrounded the anus and rectum (34). In a few reports focusing on MR imaging of mucinous adenocarcinoma (56), the mass was usually shown as having marked hyperintensity on T2-weighted images and internal mesh-like or focal solid enhancement after contrast injection. According to Shahid et al. (7), high signal intensity on T2-weighted images can be the identifying MR feature of mucinous rectal tumors for the differentiation from non-mucinous tumor. The characteristically high T2 signal intensity and mesh-like enhancement were very similar to ours. Additionally, our perianal mucinous adenocarcinoma showed new MR findings that have not been reported previously, which included an internal high T1 signal intensity comparing to that of the muscle with different signal intensity in each locule and marginal very high T1 signal intensity. The internal high signal intensity with the different signal intensity for each locule on the T1-weighted images, might result from variable proteinous content of mucin and can help to differentiate from the other types of anal tumor, as if the variable T1 signal intensity of a mass in the ovary can suggest a mucinous neoplasm (8). The finding of curvilinear high signal intensities along the margin of the some locules on T1-weighted images reflected the peripheral calcification; this phenomenon would be explained by the theory that a particular calcification could reduce the T1 relaxation time by a surface relaxation mechanism (9) and could be helpful finding to diagnose the perianal mucinous adenocarcinoma without use of CT examination.
The differential diagnosis from an epidermal/dermal cyst, tail gut cyst, sacrococcygeal teratoma, anal duct or gland cyst, cystic neurogenic cyst, and retroperitoneal pseudomyxoma involving the pelvic subperitoneum, could be difficult. However, the growth pattern of a multilocular cystic mass surrounding the anus, a high signal intensity with various signal intensity in each locule on T1-weighted images, and the peripheral/septal irregular enhancement after contrast administration can be helpful to distinguish a perianal mucinous adenocarcinoma from other tumors (4).
Although a lack of a large series or randomized trial of these tumors has led to variability in the treatment, the first treatment of choice is regarded as a surgical resection, most commonly found with an abdominoperineal resection. If the tumor can be excised and its continence retained, local excision can be an alternative. The use of neoadjuvant therapy has not been widely applied and its benefit is controversial (2). Despite aggressive surgery and adjuvant therapy, the overall prognosis of patients with a mucinous adenocarcinoma of the anal duct remains poor. In a study reported by Anthony et al. (10) the reported survival was two to 34 months in patients.
In summary, we have reported a rare case of a perianal mucinous adenocarcinoma. The tumors were manifested as a multilocular cystic mass with peripheral calcification around the anus on CT, and as an internal high T1 and T2 signal intensity with different T1 signal intensity in each locule, and peripheral/septal enhancement after contrast injection on MRI.
Figures and Tables
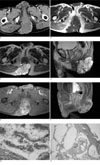
Fig. 1
An 80-year-old man with a surgically proven perianal mucinous adenocarcinoma.
A. An axial CT image after administration of contrast material shows the presence of a multilocular mass with multiple marginal calcifications. The mass is attached to the posterior aspect of the anus and extends to the skin.
B. T1-weighted MR image shows a slightly high signal intensity comparing to that of muscle, with a different internal signal intensity for each locule (small *: relatively hyposignal intensity, large *: relatively hypersignal intensity). Curvilinear high signal intensities along the margin (arrows) of some locules, which are consistent with the marginal calcification on CT, are also identified.
C, D. On the axial and sagittal T2 weighted MR images, the multilocular mass is shown as having a markedly high signal intensity and a focal invasion into the posterior aspect of the extrinsic anal sphincter is identified (arrows).
E, F. Axial and sagittal fat-saturated gadolinium-enhanced T1 weighted MR images show septal and peripheral enhancement of the tumor.
G. The multilocular cystic wall is lined by mucinous columnar cells. An overlying perianal squamous epithelial layer is seen (H & E staining, × 40).
H. The glandular epithelial cells have hyperchromatic and atypical nuclei with nuclear stratification (H & E staining, × 400).
References
1. Wong AY, Rahilly MA, Adams W, Lee CS. Mucinous anal gland carcinoma with perianal pagetoid spread. Pathology. 1998; 30:1–3.
2. Abel ME, Chiu YS, Russell TR, Volpe PA. Adenocarcinoma of the anal glands. Results of a survey. Dis Colon Rectum. 1993; 36:383–387.
3. Nishimura T, Nozue M, Suzuki K, Imai M, Suzuki S, Sakahara H, et al. Perianal mucinous carcinoma successfully treated with a combination of external beam radiotherapy and high dose rate interstitial brachytherapy. Br J Radiol. 2000; 73:661–664.
4. Yang DM, Jung DH, Kim H, Kang JH, Kim SH, Kim JH, et al. Retroperitoneal cystic masses: CT, clinical, and pathologic findings and literature review. Radiographics. 2004; 24:1353–1365.
5. Hama Y, Makita K, Yamana T, Dodanuki K. Mucinous adenocarcinoma arising from fistula in ano: MRI findings. AJR Am J Roentgenol. 2006; 187:517–521.
6. Fujimoto H, Ikeda M, Shimofusa R, Terauchi M, Eguchi M. Mucinous adenocarcinoma arising from fistula-in-ano: findings on MRI. Eur Radiol. 2003; 13:2053–2054.
7. Hussain SM, Outwater EK, Siegelman ES. Mucinous versus nonmucinous rectal carcinomas: differentiation with MR imaging. Radiology. 1999; 213:79–85.
8. Okamoto Y, Tanaka YO, Tsunoda H, Yoshikawa H, Minami M. Malignant or borderline mucinous cystic neoplasms have a larger number of loculi than mucinous cystadenoma: a retrospective study with MR. J Magn Reson Imaging. 2007; 26:94–99.
9. Henkelman RM, Watts JF, Kucharczyk W. High signal intensity in MR images of calcified brain tissue. Radiology. 1991; 179:199–206.
10. Anthony T, Simmang C, Lee EL, Turnage RH. Perianal mucinous adenocarcinoma. J Surg Oncol. 1997; 64:218–221.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download